Computational model of membrane fission catalyzed by ESCRT-III
- PMID: 19936052
- PMCID: PMC2773929
- DOI: 10.1371/journal.pcbi.1000575
Computational model of membrane fission catalyzed by ESCRT-III
Abstract
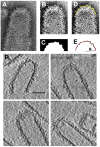
ESCRT-III proteins catalyze membrane fission during multi vesicular body biogenesis, budding of some enveloped viruses and cell division. We suggest and analyze a novel mechanism of membrane fission by the mammalian ESCRT-III subunits CHMP2 and CHMP3. We propose that the CHMP2-CHMP3 complexes self-assemble into hemi-spherical dome-like structures within the necks of the initial membrane buds generated by CHMP4 filaments. The dome formation is accompanied by the membrane attachment to the dome surface, which drives narrowing of the membrane neck and accumulation of the elastic stresses leading, ultimately, to the neck fission. Based on the bending elastic model of lipid bilayers, we determine the degree of the membrane attachment to the dome enabling the neck fission and compute the required values of the protein-membrane binding energy. We estimate the feasible values of this energy and predict a high efficiency for the CHMP2-CHMP3 complexes in mediating membrane fission. We support the computational model by electron tomography imaging of CHMP2-CHMP3 assemblies in vitro. We predict a high efficiency for the CHMP2-CHMP3 complexes in mediating membrane fission.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
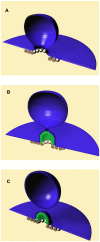

 - radius of the protein dome surface
- radius of the protein dome surface  - the neck radius,
- the neck radius,  - the attachment angle. (B) Post-fission state. The total membrane area
- the attachment angle. (B) Post-fission state. The total membrane area  .
.
 ; (2)
; (2)  ; (3)
; (3)  .
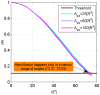
.
 whose values are presented in the insert in mN/m, the total membrane area is
whose values are presented in the insert in mN/m, the total membrane area is  .
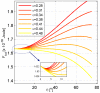
.
 ,
,  , and
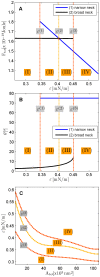
, and  . (A) The total energies of the narrow- and broad- neck configurations are represented by the lines 1 and 2, respectively. (B) The attachment angles in the narrow and broad neck configurations are represented by the lines 1 and 2, respectively,
. (A) The total energies of the narrow- and broad- neck configurations are represented by the lines 1 and 2, respectively. (B) The attachment angles in the narrow and broad neck configurations are represented by the lines 1 and 2, respectively,  (C) The characteristic affinities depending on the total membrane area
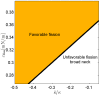
(C) The characteristic affinities depending on the total membrane area  . The phase diagrams are divided into four regions corresponding to different regimes of the possible configurations of the system: (I) only broad neck; (II) stable broad neck and quasi-stable narrow neck; (III) stable narrow neck and quasi-stable broad neck; (IV) only narrow neck.
. The phase diagrams are divided into four regions corresponding to different regimes of the possible configurations of the system: (I) only broad neck; (II) stable broad neck and quasi-stable narrow neck; (III) stable narrow neck and quasi-stable broad neck; (IV) only narrow neck.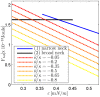
 .
.
 .
.
References
-
- McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. - PubMed
-
- Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207. - PubMed
-
- Shibata Y, Hu J, Kozlov MM, Rapoport TA. The role of membrane curvature in shaping the endoplasmic reticulum and other organelles. Ann Rev Cell Develop Biol 2009
-
- Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7:9–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

