Glypican-1 mediates both prion protein lipid raft association and disease isoform formation
- PMID: 19936054
- PMCID: PMC2773931
- DOI: 10.1371/journal.ppat.1000666
Glypican-1 mediates both prion protein lipid raft association and disease isoform formation
Abstract
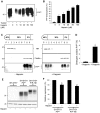
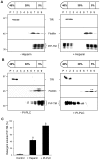
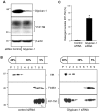
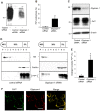
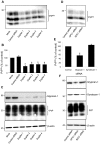
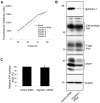
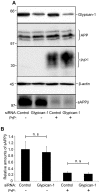
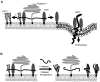
In prion diseases, the cellular form of the prion protein, PrP(C), undergoes a conformational conversion to the infectious isoform, PrP(Sc). PrP(C) associates with lipid rafts through its glycosyl-phosphatidylinositol (GPI) anchor and a region in its N-terminal domain which also binds to heparan sulfate proteoglycans (HSPGs). We show that heparin displaces PrP(C) from rafts and promotes its endocytosis, suggesting that heparin competes with an endogenous raft-resident HSPG for binding to PrP(C). We then utilised a transmembrane-anchored form of PrP (PrP-TM), which is targeted to rafts solely by its N-terminal domain, to show that both heparin and phosphatidylinositol-specific phospholipase C can inhibit its association with detergent-resistant rafts, implying that a GPI-anchored HSPG targets PrP(C) to rafts. Depletion of the major neuronal GPI-anchored HSPG, glypican-1, significantly reduced the raft association of PrP-TM and displaced PrP(C) from rafts, promoting its endocytosis. Glypican-1 and PrP(C) colocalised on the cell surface and both PrP(C) and PrP(Sc) co-immunoprecipitated with glypican-1. Critically, treatment of scrapie-infected N2a cells with glypican-1 siRNA significantly reduced PrP(Sc) formation. In contrast, depletion of glypican-1 did not alter the inhibitory effect of PrP(C) on the beta-secretase cleavage of the Alzheimer's amyloid precursor protein. These data indicate that glypican-1 is a novel cellular cofactor for prion conversion and we propose that it acts as a scaffold facilitating the interaction of PrP(C) and PrP(Sc) in lipid rafts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Glypican-1 facilitates prion conversion in lipid rafts.J Neurochem. 2011 Mar;116(5):721-5. doi: 10.1111/j.1471-4159.2010.06936.x. J Neurochem. 2011. PMID: 20681952 Review.
-
The prion protein and lipid rafts.Mol Membr Biol. 2006 Jan-Feb;23(1):89-99. doi: 10.1080/09687860500449994. Mol Membr Biol. 2006. PMID: 16611584 Review.
-
PrP Knockout Cells Expressing Transmembrane PrP Resist Prion Infection.J Virol. 2017 Jan 3;91(2):e01686-16. doi: 10.1128/JVI.01686-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27847358 Free PMC article.
-
Soluble dimeric prion protein binds PrP(Sc) in vivo and antagonizes prion disease.Cell. 2003 Apr 4;113(1):49-60. doi: 10.1016/s0092-8674(03)00201-0. Cell. 2003. PMID: 12679034
-
The role of rafts in the fibrillization and aggregation of prions.Chem Phys Lipids. 2006 Jun;141(1-2):66-71. doi: 10.1016/j.chemphyslip.2006.02.022. Epub 2006 Mar 27. Chem Phys Lipids. 2006. PMID: 16647049 Review.
Cited by
-
Modulation of Glycosaminoglycans Affects PrPSc Metabolism but Does Not Block PrPSc Uptake.J Virol. 2015 Oct;89(19):9853-64. doi: 10.1128/JVI.01276-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202247 Free PMC article.
-
Novel Polymorphisms and Genetic Characteristics of the Shadow of Prion Protein Gene (SPRN) in Cats, Hosts of Feline Spongiform Encephalopathy.Viruses. 2022 May 6;14(5):981. doi: 10.3390/v14050981. Viruses. 2022. PMID: 35632724 Free PMC article.
-
Evidence that bank vole PrP is a universal acceptor for prions.PLoS Pathog. 2014 Apr 3;10(4):e1003990. doi: 10.1371/journal.ppat.1003990. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24699458 Free PMC article.
-
The prion protein is embedded in a molecular environment that modulates transforming growth factor β and integrin signaling.Sci Rep. 2018 Jun 5;8(1):8654. doi: 10.1038/s41598-018-26685-x. Sci Rep. 2018. PMID: 29872131 Free PMC article.
-
A survey of antiprion compounds reveals the prevalence of non-PrP molecular targets.J Biol Chem. 2011 Aug 5;286(31):27718-28. doi: 10.1074/jbc.M111.234393. Epub 2011 May 24. J Biol Chem. 2011. PMID: 21610081 Free PMC article.
References
-
- Taylor DR, Hooper NM. The prion protein and lipid rafts. Mol Membr Biol. 2006;23:89–99. - PubMed
-
- Hooper NM. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae. Mol Membr Biol. 1999;16:145–156. - PubMed
-
- Baron GS, Caughey B. Effect of glycosylphosphatidylinositol anchor-dependent and -independent prion protein association with model raft membranes on conversion to the protease-resistant isoform. J Biol Chem. 2003;278:14883–14892. - PubMed
-
- Walmsley AR, Zeng F, Hooper NM. The N-terminal region of the prion protein ectodomain contains a lipid raft targeting determinant. J Biol Chem. 2003;278:37241–37248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

