An ex-vivo human intestinal model to study Entamoeba histolytica pathogenesis
- PMID: 19936071
- PMCID: PMC2777411
- DOI: 10.1371/journal.pntd.0000551
An ex-vivo human intestinal model to study Entamoeba histolytica pathogenesis
Abstract
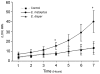
Amoebiasis (a human intestinal infection affecting 50 million people every year) is caused by the protozoan parasite Entamoeba histolytica. To study the molecular mechanisms underlying human colon invasion by E. histolytica, we have set up an ex vivo human colon model to study the early steps in amoebiasis. Using scanning electron microscopy and histological analyses, we have established that E. histolytica caused the removal of the protective mucus coat during the first two hours of incubation, detached the enterocytes, and then penetrated into the lamina propria by following the crypts of Lieberkühn. Significant cell lysis (determined by the release of lactodehydrogenase) and inflammation (marked by the secretion of pro-inflammatory molecules such as interleukin 1 beta, interferon gamma, interleukin 6, interleukin 8 and tumour necrosis factor) were detected after four hours of incubation. Entamoeba dispar (a closely related non-pathogenic amoeba that also colonizes the human colon) was unable to invade colonic mucosa, lyse cells or induce an inflammatory response. We also examined the behaviour of trophozoites in which genes coding for known virulent factors (such as amoebapores, the Gal/GalNAc lectin and the cysteine protease 5 (CP-A5), which have major roles in cell death, adhesion (to target cells or mucus) and mucus degradation, respectively) were silenced, together with the corresponding tissue responses. Our data revealed that the signalling via the heavy chain Hgl2 or via the light chain Lgl1 of the Gal/GalNAc lectin is not essential to penetrate the human colonic mucosa. In addition, our study demonstrates that E. histolytica silenced for CP-A5 does not penetrate the colonic lamina propria and does not induce the host's pro-inflammatory cytokine secretion.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Differential expression of pathogenic genes of Entamoeba histolytica vs E. dispar in a model of infection using human liver tissue explants.PLoS One. 2017 Aug 3;12(8):e0181962. doi: 10.1371/journal.pone.0181962. eCollection 2017. PLoS One. 2017. PMID: 28771523 Free PMC article.
-
[Recent findings on the pathogenicity of Entamoeba histolytica].Immun Infekt. 1992 Oct;20(5):146-50. Immun Infekt. 1992. PMID: 1452192 Review. German.
-
Recent discoveries in the pathogenesis and immune response toward Entamoeba histolytica.Future Microbiol. 2009 Feb;4(1):105-18. doi: 10.2217/17460913.4.1.105. Future Microbiol. 2009. PMID: 19207103 Review.
-
Evaluation of the C-Terminal Fragment of Entamoeba histolytica Gal/GalNAc Lectin Intermediate Subunit as a Vaccine Candidate against Amebic Liver Abscess.PLoS Negl Trop Dis. 2016 Jan 29;10(1):e0004419. doi: 10.1371/journal.pntd.0004419. eCollection 2016 Jan. PLoS Negl Trop Dis. 2016. PMID: 26824828 Free PMC article.
-
Role of various virulence factors involved in the pathogenesis of Entamoeba histolytica.Exp Parasitol. 2024 Nov;266:108841. doi: 10.1016/j.exppara.2024.108841. Epub 2024 Oct 1. Exp Parasitol. 2024. PMID: 39362393 Review.
Cited by
-
Immune Response of Amebiasis and Immune Evasion by Entamoeba histolytica.Front Immunol. 2016 May 12;7:175. doi: 10.3389/fimmu.2016.00175. eCollection 2016. Front Immunol. 2016. PMID: 27242782 Free PMC article. Review.
-
Differences in cap formation between invasive Entamoeba histolytica and non-invasive Entamoeba dispar.Parasitol Res. 2012 Jul;111(1):215-21. doi: 10.1007/s00436-012-2820-2. Epub 2012 Jan 26. Parasitol Res. 2012. PMID: 22278728
-
Regulation of Virulence of Entamoeba histolytica by the URE3-BP Transcription Factor.mBio. 2010 May 18;1(1):e00057-10. doi: 10.1128/mBio.00057-10. mBio. 2010. PMID: 20689746 Free PMC article.
-
Tissue destruction and invasion by Entamoeba histolytica.Trends Parasitol. 2011 Jun;27(6):254-63. doi: 10.1016/j.pt.2011.02.006. Epub 2011 Mar 26. Trends Parasitol. 2011. PMID: 21440507 Free PMC article. Review.
-
Effect of the leptin receptor Q223R polymorphism on the host transcriptome following infection with Entamoeba histolytica.Infect Immun. 2013 May;81(5):1460-70. doi: 10.1128/IAI.01383-12. Epub 2013 Feb 19. Infect Immun. 2013. PMID: 23429533 Free PMC article.
References
-
- Stanley SL,, Jr. Amoebiasis. Lancet. 2003;361:1025–1034. - PubMed
-
- Haque R, Mondal D, Shu J, Roy S, Kabir M, et al. Correlation of interferon-gamma production by peripheral blood mononuclear cells with childhood malnutrition and susceptibility to amebiasis. Am J Trop Med Hyg. 2007;76:340–344. - PubMed
-
- Mondal D, Petri WA,, Jr., Sack RB, Kirkpatrick BD, Haque R. Entamoeba histolytica-associated diarrheal illness is negatively associated with the growth of preschool children: evidence from a prospective study. Trans R Soc Trop Med Hyg. 2006;100:1032–1038. - PubMed
-
- Tarleton JL, Haque R, Mondal D, Shu J, Farr BM, et al. Cognitive effects of diarrhea, malnutrition, and Entamoeba histolytica infection on school age children in Dhaka, Bangladesh. Am J Trop Med Hyg. 2006;74:475–481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

