Practical lessons from theoretical models about the somitogenesis
- PMID: 19936076
- PMCID: PMC2759138
Practical lessons from theoretical models about the somitogenesis
Abstract
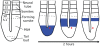
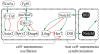
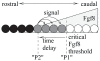
Vertebrae and other mammalian repetitive structures are formed from embryonic organs called somites. Somites arise sequentially from the unsegmented presomitic mesoderm (PSM). In mice, a new bilateral pair of somites arise every two hours from the rostral PSM. On the other hand, cells are added to the caudal side of the PSM due to cell proliferation of the tail bud. Somite formation correlates with cycles of cell-autonomous expression in the PSM of genes like Hes7. Because the somitogenesis is a highly dynamic and coordinated process, this event has been subjected to extensive theoretical modeling. Here, we describe the current understanding about the somitogenesis in mouse embryos with an emphasis on insights gained from computer simulations. It is worth noting that the combination of experiments and computer simulations has uncovered dynamical properties of the somitogenesis clock such as the transcription/translation delays, the half-life and the synchronization mechanism across the PSM. Theoretical models have also been useful to provide predictions and rigorous hypothesis about poorly understood processes such as the mechanisms by which the temporal PSM oscillations are arrested and converted into an spatial pattern. We aim at reviewing this theoretical literature in such a way that experimentalists might appreciate the resulting conclusions.
Keywords: Fgf signaling; Hes7 oscillation; Notch signaling; Theoretical models; Wnt signaling; mouse somitogenesis.
Figures

Similar articles
-
Hopf bifurcation in the presomitic mesoderm during the mouse segmentation.J Theor Biol. 2009 Jul 7;259(1):176-89. doi: 10.1016/j.jtbi.2009.02.007. Epub 2009 Feb 21. J Theor Biol. 2009. PMID: 19236883
-
Oscillatory gene expression and somitogenesis.Wiley Interdiscip Rev Dev Biol. 2012 Sep-Oct;1(5):629-41. doi: 10.1002/wdev.46. Epub 2012 Mar 22. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23799565 Review.
-
The oscillation of Notch activation, but not its boundary, is required for somite border formation and rostral-caudal patterning within a somite.Development. 2010 May;137(9):1515-22. doi: 10.1242/dev.044545. Epub 2010 Mar 24. Development. 2010. PMID: 20335362
-
Dact1 presomitic mesoderm expression oscillates in phase with Axin2 in the somitogenesis clock of mice.Dev Dyn. 2006 Nov;235(11):3177-83. doi: 10.1002/dvdy.20968. Dev Dyn. 2006. PMID: 17013874
-
A molecular clock involved in somite segmentation.Curr Top Dev Biol. 2001;51:221-48. doi: 10.1016/s0070-2153(01)51007-8. Curr Top Dev Biol. 2001. PMID: 11236715 Review.
Cited by
-
Fast synchronization of ultradian oscillators controlled by delta-notch signaling with cis-inhibition.PLoS Comput Biol. 2014 Oct 2;10(10):e1003843. doi: 10.1371/journal.pcbi.1003843. eCollection 2014 Oct. PLoS Comput Biol. 2014. PMID: 25275459 Free PMC article.
References
-
- Aulehla A, Herrmann BG. Segmentation in vertebrates: clock and gradient finally joined. Genes, Dev. 2004;18:2060–2067. - PubMed
-
- Aulehla A, Pourquié O. On periodicity and directionality of somitogenesis. Anat Embryol (Berl) 2006;211(Suppl 7):3–8. - PubMed
-
- Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, Herrmann BG. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395–406. - PubMed
-
- Baker R, Schnell S, Maini P. Formation of vertebral precursors: Past models and future predictions. Journal of Theoretical Medicine. 2003;5:23–35.
-
- Baker RE, Schnell S, Maini PK. A clock and wavefront mechanism for somite formation. Dev Biol. 2006;293:116–126. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
