Beta1-integrin signaling is essential for lens fiber survival
- PMID: 19936087
- PMCID: PMC2759130
Beta1-integrin signaling is essential for lens fiber survival
Abstract
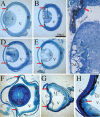
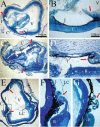
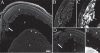
Integrins have been proposed to play a major role in lens morphogenesis. To determine the role of beta1-integrin and its down-stream signaling partner, integrin linked kinase (ILK), in lens morphogenesis, eyes of WT mice and mice with a nestin-linked conditional knockout of beta1-integrin or ILK were analyzed for defects in lens development. Mice, lacking the genes encoding the beta1-integrin subunit (Itgb1) or ILK (Ilk), showed a perinatal degeneration of the lens. Early signs of lens degeneration included vacuolization, random distribution of lens cell nuclei, disrupted fiber morphology and attenuation and separation of the lens capsule. The phenotype became progressively more severe during the first postnatal week eventually leading to the complete loss of the lens. A more severe phenotype was observed in ILK mutants at similar stages. Eyes from embryonic day 13 beta1-integrin-mutant embryos showed no obvious signs of lens degeneration, indicating that mutant lens develops normally until peri-recombination. Our findings suggest that beta1-integrins and ILK cooperate to control lens cell survival and link lens fibers to the surrounding extracellular matrix. The assembly and integrity of the lens capsule also appears to be reliant on integrin signaling within lens fibers. Extrapolation of these results indicates a novel role of integrins in lens cell-cell adhesions as well as a potential role in the pathogenesis of congenital cataracts.
Keywords: ILK; basement membrane; eye development; integrins; lens development.
Figures





Similar articles
-
Integrin-linked kinase deletion in the developing lens leads to capsule rupture, impaired fiber migration and non-apoptotic epithelial cell death.Invest Ophthalmol Vis Sci. 2012 May 17;53(6):3067-81. doi: 10.1167/iovs.11-9128. Invest Ophthalmol Vis Sci. 2012. PMID: 22491404 Free PMC article.
-
Integrin linked kinase (ILK) is required for lens epithelial cell survival, proliferation and differentiation.Exp Eye Res. 2014 Apr;121:130-42. doi: 10.1016/j.exer.2014.01.013. Epub 2014 Jan 25. Exp Eye Res. 2014. PMID: 24472646
-
The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin beta1 and activation of integrin-linked kinase.J Biol Chem. 2008 Aug 15;283(33):22826-37. doi: 10.1074/jbc.M706563200. Epub 2008 May 23. J Biol Chem. 2008. PMID: 18503049 Free PMC article.
-
Integrin-linked kinase (ILK): a "hot" therapeutic target.Biochem Pharmacol. 2000 Oct 15;60(8):1115-9. doi: 10.1016/s0006-2952(00)00444-5. Biochem Pharmacol. 2000. PMID: 11007949 Review.
-
Extracellular matrix and integrin signaling in lens development and cataract.Semin Cell Dev Biol. 2006 Dec;17(6):759-76. doi: 10.1016/j.semcdb.2006.10.006. Epub 2006 Oct 27. Semin Cell Dev Biol. 2006. PMID: 17134921 Review.
Cited by
-
Integrin-linked kinase deletion in the developing lens leads to capsule rupture, impaired fiber migration and non-apoptotic epithelial cell death.Invest Ophthalmol Vis Sci. 2012 May 17;53(6):3067-81. doi: 10.1167/iovs.11-9128. Invest Ophthalmol Vis Sci. 2012. PMID: 22491404 Free PMC article.
-
Identification of fibronectin 1 as a candidate genetic modifier in a Col4a1 mutant mouse model of Gould syndrome.Dis Model Mech. 2021 Apr 1;14(4):dmm048231. doi: 10.1242/dmm.048231. Epub 2021 Apr 26. Dis Model Mech. 2021. PMID: 34424299 Free PMC article.
-
Integrin α5/fibronectin1 and focal adhesion kinase are required for lens fiber morphogenesis in zebrafish.Mol Biol Cell. 2012 Dec;23(24):4725-38. doi: 10.1091/mbc.E12-09-0672. Epub 2012 Oct 24. Mol Biol Cell. 2012. PMID: 23097490 Free PMC article.
-
A zebrafish model of crim1 loss of function has small and misshapen lenses with dysregulated clic4 and fgf1b expression.Front Cell Dev Biol. 2025 Mar 6;13:1522094. doi: 10.3389/fcell.2025.1522094. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40114969 Free PMC article.
-
Echistatin prevents posterior capsule opacification in diabetic rabbit model via integrin linked kinase signaling pathway.Int J Clin Exp Pathol. 2015 Nov 1;8(11):14294-304. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26823745 Free PMC article.
References
-
- Aumailley M, Pesch M, Tunggal L, et al. Altered synthesis of laminin 1 and absence of basement membrane component deposition in beta-1 integrin deficient embryoid bodies. J Cell Sci. 2000;113:259–68. - PubMed
-
- Bassnett S, Missey H, Vucemilo I. Molecular architecture of the lens fiber cell basal membrane complex. J Cell Sci. 1999;112:2155–65. - PubMed
-
- Bassnett S, Winzenburger PA. Morphometric analysis of fibre cell growth in the developing avian lens. Exp Eye Res. 2003;76:291–302. - PubMed
-
- Beebe D, Garcia C, Wang X, et al. Contributions by members of the TGFbeta superfamily to lens development. Int J Dev Biol. 2004;48:845–56. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
