The coiled-coil domain of EHD2 mediates inhibition of LeEix2 endocytosis and signaling
- PMID: 19936242
- PMCID: PMC2775675
- DOI: 10.1371/journal.pone.0007973
The coiled-coil domain of EHD2 mediates inhibition of LeEix2 endocytosis and signaling
Abstract
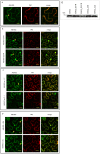
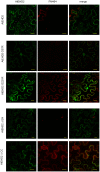
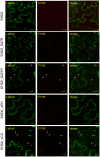
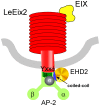
Endocytosis has been suggested to be crucial for the induction of plant immunity in several cases. We have previously shown that two Arabidopsis proteins, AtEHD1 and AtEHD2, are involved in endocytosis in plant systems. AtEHD2 has an inhibitory effect on endocytosis of transferrin, FM-4-64, and LeEix2. There are many works in mammalian systems detailing the importance of the various domains in EHDs but, to date, the domains of plant EHD2 that are required for its inhibitory activity on endocytosis remained unknown. In this work we demonstrate that the coiled-coil domain of EHD2 is crucial for the ability of EHD2 to inhibit endocytosis in plants, as mutant EHD2 forms lacking the coiled-coil lost the ability to inhibit endocytosis and signaling of LeEix2. The coiled-coil was also required for binding of EHD2 to the LeEix2 receptor. It is therefore probable that binding of EHD2 to the LeEix2 receptor is required for inhibition of LeEix2 internalization. We also show herein that the P-loop of EHD2 is important for EHD2 to function properly. The EH domain of AtEHD2 does not appear to be involved in inhibition of endocytosis. Moreover, AtEHD2 influences actin organization and may exert its inhibitory effect on endocytosis through actin re-distribution. The coiled-coil domain of EHD2 functions in inhibition of endocytosis, while the EH domain does not appear to be involved in inhibition of endocytosis.
Conflict of interest statement
Figures




Similar articles
-
The function of EHD2 in endocytosis and defense signaling is affected by SUMO.Plant Mol Biol. 2014 Mar;84(4-5):509-18. doi: 10.1007/s11103-013-0148-7. Epub 2013 Oct 24. Plant Mol Biol. 2014. PMID: 24154852
-
EHD2 inhibits ligand-induced endocytosis and signaling of the leucine-rich repeat receptor-like protein LeEix2.Plant J. 2009 Aug;59(4):600-11. doi: 10.1111/j.1365-313X.2009.03897.x. Epub 2009 Apr 20. Plant J. 2009. PMID: 19392695
-
AtEHDs in endocytosis.Plant Signal Behav. 2008 Nov;3(11):1008-10. doi: 10.4161/psb.6708. Plant Signal Behav. 2008. PMID: 19704436 Free PMC article.
-
EH domain-containing protein 2 (EHD2): Overview, biological function, and therapeutic potential.Cell Biochem Funct. 2024 Apr;42(3):e4016. doi: 10.1002/cbf.4016. Cell Biochem Funct. 2024. PMID: 38613224 Review.
-
EH and UIM: endocytosis and more.Sci STKE. 2003 Dec 16;2003(213):re17. doi: 10.1126/stke.2132003re17. Sci STKE. 2003. PMID: 14679291 Review.
Cited by
-
The MORC family: new epigenetic regulators of transcription and DNA damage response.Epigenetics. 2013 Jul;8(7):685-93. doi: 10.4161/epi.24976. Epub 2013 May 17. Epigenetics. 2013. PMID: 23804034 Free PMC article.
-
ZmEHD1 Is Required for Kernel Development and Vegetative Growth through Regulating Auxin Homeostasis.Plant Physiol. 2020 Mar;182(3):1467-1480. doi: 10.1104/pp.19.01336. Epub 2019 Dec 19. Plant Physiol. 2020. PMID: 31857426 Free PMC article.
-
Cellular functions and intrinsic attributes of the ATP-binding Eps15 homology domain-containing proteins.Protein Sci. 2020 Jun;29(6):1321-1330. doi: 10.1002/pro.3860. Epub 2020 Apr 11. Protein Sci. 2020. PMID: 32223019 Free PMC article. Review.
-
Blue-light-activated phototropin2 trafficking from the cytoplasm to Golgi/post-Golgi vesicles.J Exp Bot. 2014 Jul;65(12):3263-76. doi: 10.1093/jxb/eru172. Epub 2014 May 12. J Exp Bot. 2014. PMID: 24821953 Free PMC article.
-
EHD proteins: key conductors of endocytic transport.Trends Cell Biol. 2011 Feb;21(2):122-31. doi: 10.1016/j.tcb.2010.10.003. Epub 2010 Nov 8. Trends Cell Biol. 2011. PMID: 21067929 Free PMC article. Review.
References
-
- Murphy AS, Bandyopadhyay A, Holstein SE, Peer WA. Endocytotic cycling of PM proteins. Annu Rev Plant Biol. 2005;56:221–251. - PubMed
-
- Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nature Reviews Molecular Cell Biology. 2005;6:112–126. - PubMed
-
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. - PubMed
-
- Samaj J, Read ND, Volkmann D, Menzel D, Baluska F. The endocytic network in plants. Trends Cell Biol. 2005;15:425–433. - PubMed
-
- Kleine-Vehn J, Friml J. Polar Targeting and Endocytic Recycling in Auxin-Dependent Plant Development. Annual Review of Cell and Developmental Biology. 2008;24:447–473. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

