Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo
- PMID: 19936252
- PMCID: PMC2775917
- DOI: 10.1371/journal.pone.0007930
Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo
Abstract
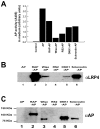
Lrp4 is a multifunctional member of the low density lipoprotein-receptor gene family and a modulator of extracellular cell signaling pathways in development. For example, Lrp4 binds Wise, a secreted Wnt modulator and BMP antagonist. Lrp4 shares structural elements within the extracellular ligand binding domain with Lrp5 and Lrp6, two established Wnt co-receptors with important roles in osteogenesis. Sclerostin is a potent osteocyte secreted inhibitor of bone formation that directly binds Lrp5 and Lrp6 and modulates both BMP and Wnt signaling. The anti-osteogenic effect of sclerostin is thought to be mediated mainly by inhibition of Wnt signaling through Lrp5/6 within osteoblasts. Dickkopf1 (Dkk1) is another potent soluble Wnt inhibitor that binds to Lrp5 and Lrp6, can displace Lrp5-bound sclerostin and is itself regulated by BMPs. In a recent genome-wide association study of bone mineral density a significant modifier locus was detected near the SOST gene at 17q21, which encodes sclerostin. In addition, nonsynonymous SNPs in the LRP4 gene were suggestively associated with bone mineral density. Here we show that Lrp4 is expressed in bone and cultured osteoblasts and binds Dkk1 and sclerostin in vitro. MicroCT analysis of Lrp4 deficient mutant mice revealed shortened total femur length, reduced cortical femoral perimeter, and reduced total femur bone mineral content (BMC) and bone mineral density (BMD). Lumbar spine trabecular bone volume per total volume (BV/TV) was significantly reduced in the mutants and the serum and urinary bone turnover markers alkaline phosphatase, osteocalcin and desoxypyridinoline were increased. We conclude that Lrp4 is a novel osteoblast expressed Dkk1 and sclerostin receptor with a physiological role in the regulation of bone growth and turnover, which is likely mediated through its function as an integrator of Wnt and BMP signaling pathways.
Conflict of interest statement
Figures




References
-
- McClung MR. The relationship between bone mineral density and fracture risk. Curr Osteoporos Rep. 2005;3:57–63. - PubMed
-
- Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. - PubMed
-
- Deal C. Potential new drug targets for osteoporosis. Nat Clin Pract Rheumatol. 2009;5:20–27. - PubMed
-
- Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

