Mycolactone gene expression is controlled by strong SigA-like promoters with utility in studies of Mycobacterium ulcerans and buruli ulcer
- PMID: 19936295
- PMCID: PMC2775157
- DOI: 10.1371/journal.pntd.0000553
Mycolactone gene expression is controlled by strong SigA-like promoters with utility in studies of Mycobacterium ulcerans and buruli ulcer
Erratum in
- PLoS Negl Trop Dis. 2010;4(2). doi: 10.1371/annotation/1e664e6f-3fc8-4586-a84e-73c4ec4f783f
Abstract
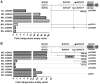
Mycolactone A/B is a lipophilic macrocyclic polyketide that is the primary virulence factor produced by Mycobacterium ulcerans, a human pathogen and the causative agent of Buruli ulcer. In M. ulcerans strain Agy99 the mycolactone polyketide synthase (PKS) locus spans a 120 kb region of a 174 kb megaplasmid. Here we have identified promoter regions of this PKS locus using GFP reporter assays, in silico analysis, primer extension, and site-directed mutagenesis. Transcription of the large PKS genes mlsA1 (51 kb), mlsA2 (7 kb) and mlsB (42 kb) is driven by a novel and powerful SigA-like promoter sequence situated 533 bp upstream of both the mlsA1 and mlsB initiation codons, which is also functional in Escherichia coli, Mycobacterium smegmatis and Mycobacterium marinum. Promoter regions were also identified upstream of the putative mycolactone accessory genes mup045 and mup053. We transformed M. ulcerans with a GFP-reporter plasmid under the control of the mls promoter to produce a highly green-fluorescent bacterium. The strain remained virulent, producing both GFP and mycolactone and causing ulcerative disease in mice. Mosquitoes have been proposed as a potential vector of M. ulcerans so we utilized M. ulcerans-GFP in microcosm feeding experiments with captured mosquito larvae. M. ulcerans-GFP accumulated within the mouth and midgut of the insect over four instars, whereas the closely related, non-mycolactone-producing species M. marinum harbouring the same GFP reporter system did not. This is the first report to identify M. ulcerans toxin gene promoters, and we have used our findings to develop M. ulcerans-GFP, a strain in which fluorescence and toxin gene expression are linked, thus providing a tool for studying Buruli ulcer pathogenesis and potential transmission to humans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- van der Werf TS, van der Graaf WT, Tappero JW, Asiedu K. Mycobacterium ulcerans infection. Lancet. 1999;354:1013–1018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

