Reelin is a platelet protein and functions as a positive regulator of platelet spreading on fibrinogen
- PMID: 19936619
- PMCID: PMC11115871
- DOI: 10.1007/s00018-009-0201-5
Reelin is a platelet protein and functions as a positive regulator of platelet spreading on fibrinogen
Abstract
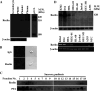
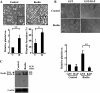
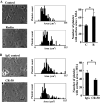
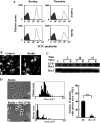
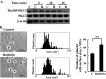
Abnormalities of platelet functions have been linked to reelin-impaired neuronal disorders. However, little attention has been given to understanding the interplay between reelin and platelet. In this study, reelin was found to present in the human platelets and megakaryocyte-like leukemic cells. Reelin-binding assays revealed that extracellular reelin can interact with platelets through the receptor belonging to the low density lipoprotein receptor gene family. The reelin-to-platelet interactions enhance platelet spreading on fibrinogen concomitant with the augmentation of lamellipodia formation and F-actin bundling. In contrast, reelin has no effect on integrin alphaIIbbeta3 activation and agonist-induced platelet aggregation. Molecular analysis revealed that the up-regulation of Rac1 activity and the inhibition of protein kinase C delta-Thr505 phosphorylation are important for reelin-mediated enhancement of platelet spreading on fibrinogen. These findings demonstrate for the first time that reelin is present in platelets and the reelin-to-platelet interactions play a novel role in platelet signaling and functions.
Figures

References
-
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/S0896-6273(00)80861-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

