Chronic administration of atypical antipsychotics improves behavioral and synaptic defects of STOP null mice
- PMID: 19936716
- PMCID: PMC2874572
- DOI: 10.1007/s00213-009-1712-3
Chronic administration of atypical antipsychotics improves behavioral and synaptic defects of STOP null mice
Abstract
Introduction: Recent studies have suggested that schizophrenia is associated with alterations in the synaptic connectivity involving cytoskeletal proteins. The microtubule-associated protein stable tubule only polypeptide (STOP) plays a key role in neuronal architecture and synaptic plasticity, and it has been demonstrated that STOP gene deletion in mice leads to a phenotype mimicking aspects of positive and negative symptoms and cognitive deficits classically observed in schizophrenic patients. In STOP null mice, behavioral defects are associated with synaptic plasticity abnormalities including defects in long-term potentiation. In these mice, long-term administration of typical antipsychotics has been shown to partially alleviate behavioral defects but, as in humans, such a treatment was poorly active on deficits related to negative symptoms and cognitive impairments. Here, we assessed the effects of risperidone and clozapine, two atypical antipsychotics, on STOP null mice behavior and synaptic plasticity.
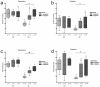
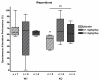
Results: Long-term administration of either drug results in alleviation of behavioral alterations mimicking some negative symptoms and partial amelioration of some cognitive defects in STOP null mice. Interestingly, clozapine treatment also improves synaptic plasticity of the STOP null animals by restoring long-term potentiation in the hippocampus.
Discussion: All together, the pharmacological reactivity of STOP null mice to antipsychotics evokes the pharmacological response of humans to such drugs. Totally, our study suggests that STOP null mice may provide a useful preclinical model to evaluate pharmacological properties of antipsychotic drugs.
Figures


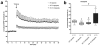
Similar articles
-
The stop null mice model for schizophrenia displays [corrected] cognitive and social deficits partly alleviated by neuroleptics.Neuroscience. 2008 Nov 11;157(1):29-39. doi: 10.1016/j.neuroscience.2008.07.080. Epub 2008 Aug 29. Neuroscience. 2008. PMID: 18804150
-
Antipsychotics increase microtubule-associated protein 2 mRNA but not spinophilin mRNA in rat hippocampus and cortex.J Neurosci Res. 2004 May 1;76(3):376-82. doi: 10.1002/jnr.20092. J Neurosci Res. 2004. PMID: 15079866
-
The suppression of brain cold-stable microtubules in mice induces synaptic defects associated with neuroleptic-sensitive behavioral disorders.Genes Dev. 2002 Sep 15;16(18):2350-64. doi: 10.1101/gad.223302. Genes Dev. 2002. PMID: 12231625 Free PMC article.
-
Discriminative stimulus properties of atypical and typical antipsychotic drugs: a review of preclinical studies.Psychopharmacology (Berl). 2009 Apr;203(2):279-94. doi: 10.1007/s00213-008-1308-3. Epub 2008 Sep 16. Psychopharmacology (Berl). 2009. PMID: 18795269 Review.
-
[Cognition, schizophrenia and the effect of antipsychotics].Encephale. 2006 May-Jun;32(3 Pt 1):341-50. doi: 10.1016/s0013-7006(06)76162-0. Encephale. 2006. PMID: 16840928 Review. French.
Cited by
-
A key function for microtubule-associated-protein 6 in activity-dependent stabilisation of actin filaments in dendritic spines.Nat Commun. 2018 Sep 17;9(1):3775. doi: 10.1038/s41467-018-05869-z. Nat Commun. 2018. PMID: 30224655 Free PMC article.
-
Altered circadian activity and sleep/wake rhythms in the stable tubule only polypeptide (STOP) null mouse model of schizophrenia.Sleep. 2021 Apr 9;44(4):zsaa237. doi: 10.1093/sleep/zsaa237. Sleep. 2021. PMID: 33186470 Free PMC article.
-
Pathophysiological Mechanisms of Antipsychotic-Induced Parkinsonism.Biomedicines. 2022 Aug 18;10(8):2010. doi: 10.3390/biomedicines10082010. Biomedicines. 2022. PMID: 36009557 Free PMC article. Review.
-
Survival Bias and Crosstalk between Chronological and Behavioral Age: Age- and Genotype-Sensitivity Tests Define Behavioral Signatures in Middle-Aged, Old, and Long-Lived Mice with Normal and AD-Associated Aging.Biomedicines. 2021 Jun 2;9(6):636. doi: 10.3390/biomedicines9060636. Biomedicines. 2021. PMID: 34199476 Free PMC article.
-
Deletion of the microtubule-associated protein 6 (MAP6) results in skeletal muscle dysfunction.Skelet Muscle. 2018 Sep 19;8(1):30. doi: 10.1186/s13395-018-0176-8. Skelet Muscle. 2018. PMID: 30231928 Free PMC article.
References
-
- Andrieux A, Salin PA, Vernet M, Kujala P, Baratier J, Gory-Fauré S, Bosc C, Pointu H, Proietto D, Schweitzer A, Denarier E, Klumperman J, Job D. The suppression of brain cold-stable microtubules in mice induces synaptic defects associated with neuroleptic-sensitive behavioral disorders. Genes Dev. 2002;16:2350–64. - PMC - PubMed
-
- Barnes TR, Curson DA, Liddle PF, Patel M. The nature and prevalence of depression in chronic schizophrenic in-patients. Br J Psychiatry. 1989;154:486–91. - PubMed
-
- Begou M, Brun P, Bertrand JB, Job D, Schweitzer A, D’Amato T, Saoud M, Andrieux A, Suaud-Chagny MF. Post-pubertal emergence of alterations in locomotor activity in stop null mice. Synapse. 2007;61:689–697. - PubMed
-
- Bégou M, Volle J, Bertrand JB, Brun P, Job D, Schweitzer A, Saoud M, D’Amato T, Andrieux A, Suaud-Chagny MF. The stop null mice model for schizophrenia displays cognitive and social deficits partly alleviated by neuroleptics. Neuroscience. 2008;157:29–39. - PubMed
-
- Blackwood DH, Pickard BJ, Thomson PA, Evans KL, Porteous DJ, Muir WJ. Are some genetic risk factors common to schizophrenia, bipolar disorder and depression? Evidence from DISC1, GRIK4 and NRG1. Neurotox Res. 2007;11:73–83. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

