Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study
- PMID: 19937043
- PMCID: PMC2916065
- DOI: 10.1007/s00401-009-0615-z
Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study
Abstract
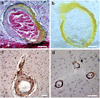
Neuroimaging with iron-sensitive MR sequences [gradient echo T2* and susceptibility-weighted imaging (SWI)] identifies small signal voids that are suspected brain microbleeds. Though the clinical significance of these lesions remains uncertain, their distribution and prevalence correlates with cerebral amyloid angiopathy (CAA), hypertension, smoking, and cognitive deficits. Investigation of the pathologies that produce signal voids is necessary to properly interpret these imaging findings. We conducted a systematic correlation of SWI-identified hypointensities to tissue pathology in postmortem brains with Alzheimer’s disease (AD) and varying degrees of CAA. Autopsied brains from eight AD patients, six of which showed advanced CAA, were imaged at 3T; foci corresponding to hypointensities were identified and studied histologically. A variety of lesions was detected; the most common lesions were acute microhemorrhage, hemosiderin residua of old hemorrhages, and small lacunes ringed by hemosiderin. In lesions where the bleeding vessel could be identified, β-amyloid immunohistochemistry confirmed the presence of β-amyloid in the vessel wall. Significant cellular apoptosis was noted in the perifocal region of recent bleeds along with heme oxygenase 1 activity and late complement activation. Acutely extravasated blood and hemosiderin were noted to migrate through enlarged Virchow–Robin spaces propagating an inflammatory reaction along the local microvasculature; a mechanism that may contribute to the formation of lacunar infarcts. Correlation of imaging findings to tissue pathology in our cases indicates that a variety of CAA-related pathologies produce MR-identified signal voids and further supports the use of SWI as a biomarker for this disease.
Figures





References
-
- Anders K, Wang Z, Kornfeld M, et al. Giant cell arteritis in association with cerebral amyloid angiopathy: immunohistochemical and molecular studies. Hum Pathol. 1997;89:1237–1246. - PubMed
-
- Atlas SW, Mark AS, Grossman RI, Gomori JM. Intracranial hemorrhage: gradient-echo MR imaging at 1.5 T. Comparison with spin-echo imaging and clinical applications. Radiology. 1988;168:803–807. - PubMed
-
- Blamire A, Rowe J, Styles P, McDonald B. Optimising imaging parameters for post mortem MR imaging of the human brain. Acta Radiol. 1999;40:593–597. - PubMed
-
- Bush V, Moyer T, Batts K, Parisi J. Essential and toxic element concentrations in fresh and formalin-fixed human autopsy tissues. Clin Chem. 1995;41(2):284–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

