beta-Strand interactions at the domain interface critical for the stability of human lens gammaD-crystallin
- PMID: 19937657
- PMCID: PMC2817848
- DOI: 10.1002/pro.296
beta-Strand interactions at the domain interface critical for the stability of human lens gammaD-crystallin
Abstract
Human age-onset cataracts are believed to be caused by the aggregation of partially unfolded or covalently damaged lens crystallin proteins; however, the exact molecular mechanism remains largely unknown. We have used microseconds of molecular dynamics simulations with explicit solvent to investigate the unfolding process of human lens gammaD-crystallin protein and its isolated domains. A partially unfolded folding intermediate of gammaD-crystallin is detected in simulations with its C-terminal domain (C-td) folded and N-terminal domain (N-td) unstructured, in excellent agreement with biochemical experiments. Our simulations strongly indicate that the stability and the folding mechanism of the N-td are regulated by the interdomain interactions, consistent with experimental observations. A hydrophobic folding core was identified within the C-td that is comprised of a and b strands from the Greek key motif 4, the one near the domain interface. Detailed analyses reveal a surprising non-native surface salt-bridge between Glu135 and Arg142 located at the end of the ab folded hairpin turn playing a critical role in stabilizing the folding core. On the other hand, an in silico single E135A substitution that disrupts this non-native Glu135-Arg142 salt-bridge causes significant destabilization to the folding core of the isolated C-td, which, in turn, induces unfolding of the N-td interface. These findings indicate that certain highly conserved charged residues, that is, Glu135 and Arg142, of gammaD-crystallin are crucial for stabilizing its hydrophobic domain interface in native conformation, and disruption of charges on the gammaD-crystallin surface might lead to unfolding and subsequent aggregation.
Figures
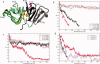
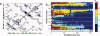


References
-
- Pandey SK, Apple DJ, Werner L, Maloof AJ, Milverton EJ. Posterior capsule opacification: a review of the aetiopathogenesis, experimental and clinical studies and factors for prevention. Indian J Ophthalmol. 2004;52:99–112. - PubMed
-
- Benedek GB. Cataract as a protein condensation disease: the Proctor Lecture. Invest Ophthalmol Vis Sci. 1997;38:1911–1921. - PubMed
-
- Aarts HJM, Lubsen NH, Schoenmakers JGG. Crystallin gene-expression during rat lens development. Eur J Biochem. 1989;183:31–36. - PubMed
-
- Lampi KJ, Shih M, Ueda Y, Shearer TR, David LL. Lens proteomics: analysis of rat crystallin sequences and two-dimensional electrophoresis map. Invest Ophthalmol Visual Sci. 2002;43:216–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

