Strain release in C-H bond activation?
- PMID: 19937877
- PMCID: PMC2879134
- DOI: 10.1002/anie.200904474
Strain release in C-H bond activation?
Abstract
What a relief! In 1955 the principle of strain release was put forward to explain the differing reactivity of axial and equitorial alcohols during oxidation. Our findings suggest that this same rationale may account for the differing rates of activation between axial and equitorial C–H bonds in C–H activation processes. In conjunction with steric and electronic considerations, strain-release can be used to qualitatively predict relative rates and site specificity of C–H activation in complex settings.
Figures

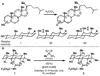
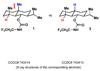
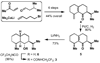
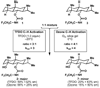


References
-
-
For reviews, see Crabtree RH. J. Chem. Soc. Dalton Trans. 2001:2437–2450. Labinger JA, Bercaw JE. Nature. 2002;417:507–514. Dick AR, Sanford MS. Tetrahedron. 2006;62:2439–2463. Godula K, Sames D. Science. 2006;312:67–72. Davies HML, Manning JR. Nature. 2008;451:417–424. Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem. Int. Ed. 2009;48:5094–5115., and references cited therein.
-
-
-
See reference , and the following reports: Seyferth D, Cheng YM. J. Am. Chem. Soc. 1973;95:6763–6770. Barton DHR, Basu NK, Day MJ, Hesse RH, Perchet MM, Starratt AN. J. Chem. Soc. Perkin Trans. I. 1975:2243–2251. Bovicelli P, Lupattelli P, Mincione E. J. Org. Chem. 1992;57:5052–5054. Chen H, Schlecht S, Semple TC, Hartwig JF. Science. 2000;287:1995–1997. González-Núnez ME, Castellano G, Andreu C, Royo J, Báguena M, Mello R, Asensio G. J. Am. Chem. Soc. 2001;123:7487–7491. Davies HML, Antoulinakis EG. J. Organomet. Chem. 2001;617:47–55. 3.20618. Yang J, Gabriele B, Belvedere S, Huang Y, Breslow R. J. Org. Chem. 2002;67:5057–5067. Francisco CG, Herrera AJ, Suárez E. J. Org. Chem. 2003;68:1012–1017. Díaz-Requejo MM, Belderrain TR, Nicasio MC, Pérez PJ. Dalton Trans. 2006:5559–5566. Daugulis O, Zaitsev VG, Shabashov D, Pham Q, Lazareva A. Synlett. 2006;20:3382–3388. Company A, Gómez L, Güell M, Ribas X, Luis JM, Que L, Jr, Costas M. J. Am. Chem. Soc. 2007;129:15766–15767. Fiori KW, Du Bois J. J. Am. Chem. Soc. 2007;129:562–568. Chen MS, White MC. Science. 2007;318:783–787. Kurokawa T, Kim M, Du Bois J. Angew. Chem. Int. Ed. 2009;48:2777–2779., and references cited therein.
-
-
- Varkony H, Pass S, Mazur Y. J. Chem. Soc. Chem. Comm. 1974:437–438.
- Schneider H-J, Müller W. J. Org. Chem. 1985;50:4609–4615.
- Mello R, Fiorentino M, Fusco C, Curci R. J. Am. Chem. Soc. 1989;111:6749–6757.
- Likhotvorik IR, Yuan K, Brown DW, Krasutsky PA, Smyth N, Jones M., Jr Tetrahedron Lett. 1992;33:911–914.
- Chen K, Que L., Jr J. Am. Chem. Soc. 2001;123:6327–6337. - PubMed
- Gómez L, Garcia-Bosch I, Company A, Benet-Buchholz J, Polo A, Sala X, Ribas X, Costas M. Angew. Chem. Int. Ed. 2009;48:5720–5723. - PubMed
-
- Chen K, Baran PS. Nature. 2009;459:824–828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

