Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica
- PMID: 19938104
- PMCID: PMC3180961
- DOI: 10.1002/ana.21802
Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica
Abstract
Objective: The serum of most neuromyelitis optica (NMO) patients contains autoantibodies (NMO-IgGs) directed against the aquaporin-4 (AQP4) water channel located on astrocyte foot processes in the perivessel and subpial areas of the brain. Our objectives were to determine the source of central nervous system (CNS) NMO-IgGs and their role in disease pathogenesis.
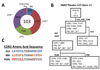
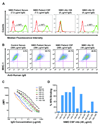
Methods: Fluorescence-activated cell sorting and single-cell reverse transcriptase polymerase chain reaction were used to identify overrepresented plasma cell immunoglobulin (Ig) sequences in the cerebrospinal fluid (CSF) of an NMO patient after a first clinical attack. Monoclonal recombinant antibodies (rAbs) were generated from the paired heavy and light chain sequences and tested for target specificity and Fc effector function. The effect of CSF rAbs on CNS immunopathology was investigated by delivering single rAbs to rats with experimental autoimmune encephalomyelitis (EAE).
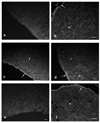
Results: Repertoire analysis revealed a dynamic, clonally expanded plasma cell population with features of an antigen-targeted response. Using multiple independent assays, 6 of 11 rAbs generated from CSF plasma cell clones specifically bound to AQP4. AQP4-specific rAbs recognized conformational epitopes and mediated both AQP4-directed antibody-dependent cellular cytotoxicity and complement-mediated lysis. When administered to rats with EAE, an AQP4-specific NMO CSF rAb induced NMO immunopathology: perivascular astrocyte depletion, myelinolysis, and complement and Ig deposition.
Interpretation: Molecular characterization of the CSF plasma cell repertoire in an early NMO patient demonstrates that AQP4-specific Ig is synthesized intrathecally at disease onset and directly contributes to CNS pathology. AQP4 is now the first confirmed antigenic target in human demyelinating disease.
Conflict of interest statement
Figures



Comment in
-
Have we finally identified an autoimmune demyelinating disease?Ann Neurol. 2009 Nov;66(5):572-3. doi: 10.1002/ana.21838. Ann Neurol. 2009. PMID: 19938069 No abstract available.
-
Have we finally identified an autoimmune demyelinating disease?Ann Neurol. 2010 Apr;67(4):552; author reply 552-3. doi: 10.1002/ana.21990. Ann Neurol. 2010. PMID: 20437595 No abstract available.
References
-
- Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet neurology. 2007;6:805–815. - PubMed
-
- Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. - PubMed
-
- Roemer SF, Parisi JE, Lennon VA, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. - PubMed
-
- Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130:1235–1243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

