Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology
- PMID: 19939208
- PMCID: PMC2925289
- DOI: 10.1089/ars.2009.2657
Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology
Abstract
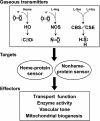
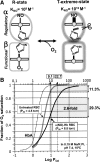
The diverse physiological actions of the "biologic gases," O2, CO, NO, and H2S, have attracted much interest. Initially viewed as toxic substances, CO, NO, and H2S play important roles as signaling molecules. The multiplicity of gas actions and gas targets and the difficulty in measuring local gas concentrations obscures detailed mechanisms whereby gases exert their actions, and many questions remain unanswered. It is now readily apparent, however, that heme-based proteins play central roles in gas-generation/reception mechanisms and provide a point where multiple gases can interact. In this review, we consider a number of key issues related to "gas biology," including the effective tissue concentrations of these gases and the importance and significance of the physical proximity of gas-producing and gas-receptor/sensors. We also take an integrated approach to the interaction of gases by considering the physiological significance of CO, NO, and H2S on mitochondrial cytochrome c oxidase, a key target and central mediator of mitochondrial respiration. Additionally, we consider the effects of biologic gases on mitochondrial biogenesis and "suspended animation." By evaluating gas-mediated control functions from both in vitro and in vivo perspectives, we hope to elaborate on the complex multiple interactions of O2, NO, CO, and H2S.
Figures





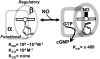



References
-
- Abu-Soud HM. Wang J. Rousseau DL. Fukuto JM. Ignarro LJ. Stuehr DJ. Neuronal nitric oxide synthase self-inactivates by forming a ferrous-nitrosyl complex during aerobic catalysis. J Biol Chem. 1995;270:22997–23006. - PubMed
-
- Adachi T. Ishikawa K. Hida W. Matsumoto H. Masuda T. Date F. Ogawa K. Takeda K. Furuyama K. Zhang Y. Kitamuro T. Ogawa H. Maruyama Y. Shibahara S. Hypoxemia and blunted hypoxic ventilatory responses in mice lacking heme oxygenase-2. Biochem Biophys Res Commun. 2004;320:514–522. - PubMed
-
- Ahmad AS. Zhuang H. Dore S. Heme oxygenase-1 protects brain from acute excitotoxicity. Neuroscience. 2006;141:1703–1708. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

