Cellular corepressor TLE2 inhibits replication-and-transcription- activator-mediated transactivation and lytic reactivation of Kaposi's sarcoma-associated herpesvirus
- PMID: 19939918
- PMCID: PMC2812399
- DOI: 10.1128/JVI.01984-09
Cellular corepressor TLE2 inhibits replication-and-transcription- activator-mediated transactivation and lytic reactivation of Kaposi's sarcoma-associated herpesvirus
Abstract
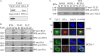
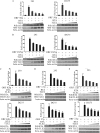
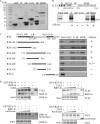
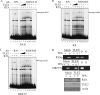
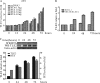
Replication and transcription activator (RTA) encoded by open reading frame 50 (ORF50) of Kaposi's sarcoma-associated herpesvirus (KSHV) is essential and sufficient to initiate lytic reactivation. RTA activates its target genes through direct binding with high affinity to its responsive elements or by interaction with cellular factors, such as RBP-Jkappa, Ap-1, C/EBP-alpha, and Oct-1. In this study, we identified transducin-like enhancer of split 2 (TLE2) as a novel RTA binding protein by using yeast two-hybrid screening of a human spleen cDNA library. The interaction between TLE2 and RTA was confirmed by glutathione S-transferase (GST) binding and coimmunoprecipitation assays. Immunofluorescence analysis showed that TLE2 and RTA were colocalized in the same nuclear compartment in KSHV-infected cells. This interaction recruited TLE2 to RTA bound to its recognition sites on DNA and repressed RTA auto-activation and transactivation activity. Moreover, TLE2 also inhibited the induction of lytic replication and virion production driven by RTA. We further showed that the Q (Gln-rich), SP (Ser-Pro-rich), and WDR (Trp-Asp repeat) domains of TLE2 and the Pro-rich domain of RTA were essential for this interaction. RBP-Jkappa has been shown previously to bind to the same Pro-rich domain of RTA, and this binding can be subject to competition by TLE2. In addition, TLE2 can form a complex with RTA to access the cognate DNA sequence of the RTA-responsive element at different promoters. Intriguingly, the transcription level of TLE2 could be upregulated by RTA during the lytic reactivation process. In conclusion, we identified a new RTA binding protein, TLE2, and demonstrated that TLE2 inhibited replication and transactivation mediated by RTA. This provides another potentially important mechanism for maintenance of KSHV viral latency through interaction with a host protein.
Figures
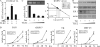

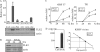

References
-
- Ashraf, S. I., and Y. T. Ip. 1998. Transcriptional control: repression by local chromatin modification. Curr. Biol. 8:R683-R686. - PubMed
-
- Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. - PubMed
-
- Bernard, S., B. Cajavec, L. Pujo-Menjouet, M. C. Mackey, and H. Herzel. 2006. Modelling transcriptional feedback loops: the role of Gro/TLE1 in Hes1 oscillations. Philos. Transact. A Math. Phys. Eng. Sci. 364:1155-1170. - PubMed
-
- Buscarlet, M., and S. Stifani. 2007. The “Marx” of Groucho on development and disease. Trends Cell Biol. 17:353-361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

