Pandemic H1N1 2009 influenza A virus induces weak cytokine responses in human macrophages and dendritic cells and is highly sensitive to the antiviral actions of interferons
- PMID: 19939920
- PMCID: PMC2812319
- DOI: 10.1128/JVI.01619-09
Pandemic H1N1 2009 influenza A virus induces weak cytokine responses in human macrophages and dendritic cells and is highly sensitive to the antiviral actions of interferons
Abstract
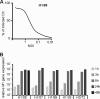
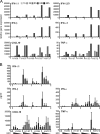
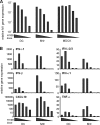
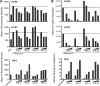
In less than 3 months after the first cases of swine origin 2009 influenza A (H1N1) virus infections were reported from Mexico, WHO declared a pandemic. The pandemic virus is antigenically distinct from seasonal influenza viruses, and the majority of human population lacks immunity against this virus. We have studied the activation of innate immune responses in pandemic virus-infected human monocyte-derived dendritic cells (DC) and macrophages. Pandemic A/Finland/553/2009 virus, representing a typical North American/European lineage virus, replicated very well in these cells. The pandemic virus, as well as the seasonal A/Brisbane/59/07 (H1N1) and A/New Caledonia/20/99 (H1N1) viruses, induced type I (alpha/beta interferon [IFN-alpha/beta]) and type III (IFN-lambda1 to -lambda3) IFN, CXCL10, and tumor necrosis factor alpha (TNF-alpha) gene expression weakly in DCs. Mouse-adapted A/WSN/33 (H1N1) and human A/Udorn/72 (H3N2) viruses, instead, induced efficiently the expression of antiviral and proinflammatory genes. Both IFN-alpha and IFN-beta inhibited the replication of the pandemic (H1N1) virus. The potential of IFN-lambda3 to inhibit viral replication was lower than that of type I IFNs. However, the pandemic virus was more sensitive to the antiviral IFN-lambda3 than the seasonal A/Brisbane/59/07 (H1N1) virus. The present study demonstrates that the novel pandemic (H1N1) influenza A virus can readily replicate in human primary DCs and macrophages and efficiently avoid the activation of innate antiviral responses. It is, however, highly sensitive to the antiviral actions of IFNs, which may provide us an additional means to treat severe cases of infection especially if significant drug resistance emerges.
Figures
Similar articles
-
In vitro anti-influenza A activity of interferon (IFN)-λ1 combined with IFN-β or oseltamivir carboxylate.Antiviral Res. 2014 Nov;111:112-20. doi: 10.1016/j.antiviral.2014.09.008. Epub 2014 Sep 20. Antiviral Res. 2014. PMID: 25245230
-
Differential Modulation of Innate Immune Responses in Human Primary Cells by Influenza A Viruses Carrying Human or Avian Nonstructural Protein 1.J Virol. 2019 Dec 12;94(1):e00999-19. doi: 10.1128/JVI.00999-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597767 Free PMC article.
-
Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells.J Virol. 2005 Aug;79(15):9608-17. doi: 10.1128/JVI.79.15.9608-9617.2005. J Virol. 2005. PMID: 16014923 Free PMC article.
-
[Drug-resistant influenza viruses: an overview].Nihon Rinsho. 2010 Sep;68(9):1671-8. Nihon Rinsho. 2010. PMID: 20845746 Review. Japanese.
-
Molecular characteristics of the human pandemic influenza A virus (H1N1).Acta Virol. 2010;54(3):155-63. doi: 10.4149/av_2010_03_155. Acta Virol. 2010. PMID: 20822307 Review.
Cited by
-
Adjuvantation of an Influenza Hemagglutinin Antigen with TLR4 and NOD2 Agonists Encapsulated in Poly(D,L-Lactide-Co-Glycolide) Nanoparticles Enhances Immunogenicity and Protection against Lethal Influenza Virus Infection in Mice.Vaccines (Basel). 2020 Sep 10;8(3):519. doi: 10.3390/vaccines8030519. Vaccines (Basel). 2020. PMID: 32927915 Free PMC article.
-
Nonpathogenic Lactobacillus rhamnosus activates the inflammasome and antiviral responses in human macrophages.Gut Microbes. 2012 Nov-Dec;3(6):510-22. doi: 10.4161/gmic.21736. Epub 2012 Aug 16. Gut Microbes. 2012. PMID: 22895087 Free PMC article.
-
Cytokine production by primary human macrophages infected with highly pathogenic H5N1 or pandemic H1N1 2009 influenza viruses.J Gen Virol. 2011 Jun;92(Pt 6):1428-1434. doi: 10.1099/vir.0.030346-0. Epub 2011 Mar 2. J Gen Virol. 2011. PMID: 21367984 Free PMC article.
-
Infection with highly pathogenic H7 influenza viruses results in an attenuated proinflammatory cytokine and chemokine response early after infection.J Infect Dis. 2011 Jan 1;203(1):40-8. doi: 10.1093/infdis/jiq018. J Infect Dis. 2011. PMID: 21148495 Free PMC article.
-
IFNAR2-dependent gene expression profile induced by IFN-α in Pteropus alecto bat cells and impact of IFNAR2 knockout on virus infection.PLoS One. 2017 Aug 9;12(8):e0182866. doi: 10.1371/journal.pone.0182866. eCollection 2017. PLoS One. 2017. PMID: 28793350 Free PMC article.
References
-
- Cantell, K., and J. Pirhonen. 1996. IFN-gamma enhances production of IFN-alpha in human macrophages but not in monocytes. J. Interferon Cytokine Res. 16:461-463. - PubMed
-
- Coccia, E. M., M. Severa, E. Giacomini, D. Monneron, M. E. Remoli, I. Julkunen, M. Cella, R. Lande, and G. Uze. 2004. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur. J. Immunol. 34:796-805. - PubMed
-
- Dawood, F. S., S. Jain, L. Finelli, M. W. Shaw, S. Lindstrom, R. J. Garten, L. V. Gubareva, X. Xu, C. B. Bridges, and T. M. Uyeki. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605-2615. - PubMed
-
- de Jong, M. D., C. P. Simmons, T. T. Thanh, V. M. Hien, G. J. Smith, T. N. Chau, D. M. Hoang, N. V. Chau, T. H. Khanh, V. C. Dong, P. T. Qui, B. V. Cam, Q. Ha do, Y. Guan, J. S. Peiris, N. T. Chinh, T. T. Hien, and J. Farrar. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12:1203-1207. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

