Circadian regulation of a-type potassium currents in the suprachiasmatic nucleus
- PMID: 19939959
- PMCID: PMC2822681
- DOI: 10.1152/jn.00670.2009
Circadian regulation of a-type potassium currents in the suprachiasmatic nucleus
Abstract
In mammals, the precise circadian timing of many biological processes depends on the generation of oscillations in neural activity of pacemaker cells in the suprachiasmatic nucleus (SCN) of the hypothalamus. Understanding the ionic mechanisms underlying these rhythms is an important goal of research in chronobiology. Previous work has shown that SCN neurons express A-type potassium currents (IAs), but little is known about the properties of this current in the SCN. We sought to characterize some of these properties, including the identities of IA channel subunits found in the SCN and the circadian regulation of IA itself. In this study, we were able to detect significant hybridization for Shal-related family members 1 and 2 (Kv4.1 and 4.2) within the SCN. In addition, we used Western blot to show that the Kv4.1 and 4.2 proteins are expressed in SCN tissue. We further show that the magnitude of the IA current exhibits a diurnal rhythm that peaks during the day in the dorsal region of the mouse SCN. This rhythm seems to be driven by a subset of SCN neurons with a larger peak current and a longer decay constant. Importantly, this rhythm in neurons in the dorsal SCN continues in constant darkness, providing an important demonstration of the circadian regulation of an intrinsic voltage-gated current in mammalian cells. We conclude that the anatomical expression, biophysical properties, and pharmacological profiles measured are all consistent with the SCN IA current being generated by Kv4 channels. Additionally, these data suggest a role for IA in the regulation of spontaneous action potential firing during the transitions between day/night and in the integration of synaptic inputs to SCN neurons throughout the daily cycle.
Figures




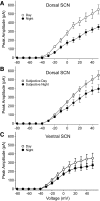
 ) and night (•). Activity onset is defined as CT 12. C: IA currents recorded in the vSCN during the day were not significantly different from during the night. Each current-voltage relationship consists of data collected from 10 to 17 neurons. Markers and error bars represent means ± SE.
) and night (•). Activity onset is defined as CT 12. C: IA currents recorded in the vSCN during the day were not significantly different from during the night. Each current-voltage relationship consists of data collected from 10 to 17 neurons. Markers and error bars represent means ± SE.
Similar articles
-
IA Channels Encoded by Kv1.4 and Kv4.2 Regulate Circadian Period of PER2 Expression in the Suprachiasmatic Nucleus.J Biol Rhythms. 2015 Oct;30(5):396-407. doi: 10.1177/0748730415593377. Epub 2015 Jul 6. J Biol Rhythms. 2015. PMID: 26152125 Free PMC article.
-
I(A) channels encoded by Kv1.4 and Kv4.2 regulate neuronal firing in the suprachiasmatic nucleus and circadian rhythms in locomotor activity.J Neurosci. 2012 Jul 18;32(29):10045-52. doi: 10.1523/JNEUROSCI.0174-12.2012. J Neurosci. 2012. PMID: 22815518 Free PMC article.
-
Fast delayed rectifier potassium current is required for circadian neural activity.Nat Neurosci. 2005 May;8(5):650-6. doi: 10.1038/nn1448. Epub 2005 Apr 24. Nat Neurosci. 2005. PMID: 15852012 Free PMC article.
-
Ion Channels Controlling Circadian Rhythms in Suprachiasmatic Nucleus Excitability.Physiol Rev. 2020 Oct 1;100(4):1415-1454. doi: 10.1152/physrev.00027.2019. Epub 2020 Mar 12. Physiol Rev. 2020. PMID: 32163720 Free PMC article. Review.
-
Linking neural activity and molecular oscillations in the SCN.Nat Rev Neurosci. 2011 Sep 2;12(10):553-69. doi: 10.1038/nrn3086. Nat Rev Neurosci. 2011. PMID: 21886186 Free PMC article. Review.
Cited by
-
Blunted diurnal firing in lateral habenula projections to dorsal raphe nucleus and delayed photoentrainment in stress-susceptible mice.PLoS Biol. 2021 Mar 10;19(3):e3000709. doi: 10.1371/journal.pbio.3000709. eCollection 2021 Mar. PLoS Biol. 2021. PMID: 33690628 Free PMC article.
-
Kv12-Encoded K + Channels Drive the Day-Night Switch in the Repetitive Firing Rates of SCN Neurons.bioRxiv [Preprint]. 2023 Feb 2:2023.01.30.526323. doi: 10.1101/2023.01.30.526323. bioRxiv. 2023. Update in: J Gen Physiol. 2023 Sep 4;155(9):e202213310. doi: 10.1085/jgp.202213310. PMID: 36778242 Free PMC article. Updated. Preprint.
-
Deficient AMPK activity contributes to hyperexcitability in peripheral nociceptive sensory neurons and thermal hyperalgesia in lupus mice.PLoS One. 2023 Jul 13;18(7):e0288356. doi: 10.1371/journal.pone.0288356. eCollection 2023. PLoS One. 2023. PMID: 37440542 Free PMC article.
-
RNA editing of the IQ domain in Ca(v)1.3 channels modulates their Ca²⁺-dependent inactivation.Neuron. 2012 Jan 26;73(2):304-16. doi: 10.1016/j.neuron.2011.11.022. Neuron. 2012. PMID: 22284185 Free PMC article.
-
Fibroblast circadian rhythms of PER2 expression depend on membrane potential and intracellular calcium.Chronobiol Int. 2012 Jul;29(6):653-64. doi: 10.3109/07420528.2012.679330. Chronobiol Int. 2012. PMID: 22734566 Free PMC article.
References
-
- Albus H, Bonnefont X, Chaves I, Yasui A, Doczy J, van der Horst GT, Meijer JH. Cryoptochrome-deficient mice lack circadian electrical activity in the suprachiasmatic nuclei. Curr Biol 12: 1130–1133, 2002 - PubMed
-
- Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci 28: 145–151, 2005 - PubMed
-
- Baranauskas G. Ionic channel function in action potential generation: current perspective. Mol Neurobiol 35: 129–150, 2007 - PubMed
-
- Belle MDC, Diekman CO, Forger DB, Piggins HD. Daily electrical silencing in the mammalian circadian clock. Science 326: 281–284, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

