Role of supplementary eye field in saccade initiation: executive, not direct, control
- PMID: 19939963
- PMCID: PMC2822692
- DOI: 10.1152/jn.00221.2009
Role of supplementary eye field in saccade initiation: executive, not direct, control
Abstract
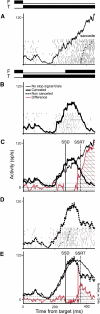
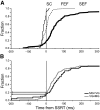
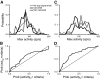
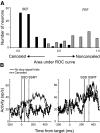
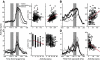
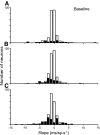
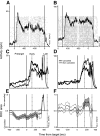
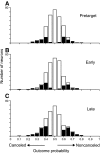
The goal of this study was to determine whether the activity of neurons in the supplementary eye field (SEF) is sufficient to control saccade initiation in macaque monkeys performing a saccade countermanding (stop signal) task. As previously observed, many neurons in the SEF increase the discharge rate before saccade initiation. However, when saccades are canceled in response to a stop signal, effectively no neurons with presaccadic activity display discharge rate modulation early enough to contribute to saccade cancellation. Moreover, SEF neurons do not exhibit a specific threshold discharge rate that could trigger saccade initiation. Yet, we observed more subtle relations between SEF activation and saccade production. The activity of numerous SEF neurons was correlated with response time and varied with sequential adjustments in response latency. Trials in which monkeys canceled or produced a saccade in a stop signal trial were distinguished by a modest difference in discharge rate of these SEF neurons before stop signal or target presentation. These findings indicate that neurons in the SEF, in contrast to counterparts in the frontal eye field and superior colliculus, do not contribute directly and immediately to the initiation of visually guided saccades. However the SEF may proactively regulate saccade production by biasing the balance between gaze-holding and gaze-shifting based on prior performance and anticipated task requirements.
Figures
Similar articles
-
Relationship of presaccadic activity in frontal eye field and supplementary eye field to saccade initiation in macaque: Poisson spike train analysis.Exp Brain Res. 1995;103(1):85-96. doi: 10.1007/BF00241967. Exp Brain Res. 1995. PMID: 7615040
-
Supplementary eye field: representation of saccades and relationship between neural response fields and elicited eye movements.J Neurophysiol. 2000 Nov;84(5):2605-21. doi: 10.1152/jn.2000.84.5.2605. J Neurophysiol. 2000. PMID: 11068002
-
Supplementary eye field encodes option and action value for saccades with variable reward.J Neurophysiol. 2010 Nov;104(5):2634-53. doi: 10.1152/jn.00430.2010. Epub 2010 Aug 25. J Neurophysiol. 2010. PMID: 20739596 Free PMC article.
-
Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: comparison with supplementary eye fields.J Neurophysiol. 1991 Aug;66(2):559-79. doi: 10.1152/jn.1991.66.2.559. J Neurophysiol. 1991. PMID: 1774586
-
Executive control of gaze by the frontal lobes.Cogn Affect Behav Neurosci. 2007 Dec;7(4):396-412. doi: 10.3758/cabn.7.4.396. Cogn Affect Behav Neurosci. 2007. PMID: 18189013 Review.
Cited by
-
Functional architecture of executive control and associated event-related potentials in macaques.Nat Commun. 2022 Oct 21;13(1):6270. doi: 10.1038/s41467-022-33942-1. Nat Commun. 2022. PMID: 36271051 Free PMC article.
-
Proactive and reactive control by the medial frontal cortex.Front Neuroeng. 2012 Jun 19;5:9. doi: 10.3389/fneng.2012.00009. eCollection 2012. Front Neuroeng. 2012. PMID: 22723779 Free PMC article.
-
Covariations between pupil diameter and supplementary eye field activity suggest a role in cognitive effort implementation.PLoS Biol. 2022 May 26;20(5):e3001654. doi: 10.1371/journal.pbio.3001654. eCollection 2022 May. PLoS Biol. 2022. PMID: 35617290 Free PMC article.
-
Computerized Eye-Tracking Training Improves the Saccadic Eye Movements of Children with Attention-Deficit/Hyperactivity Disorder.Brain Sci. 2020 Dec 21;10(12):1016. doi: 10.3390/brainsci10121016. Brain Sci. 2020. PMID: 33371236 Free PMC article.
-
Neural processes of preparatory control for stop signal inhibition.Hum Brain Mapp. 2012 Dec;33(12):2785-96. doi: 10.1002/hbm.21399. Epub 2011 Oct 5. Hum Brain Mapp. 2012. PMID: 21976392 Free PMC article.
References
-
- Amador N, Schlag-Rey M, Schlag J. Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. J Neurophysiol 84: 2166–2170, 2000 - PubMed
-
- Amador N, Schlag-Rey M, Schlag J. Primate antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. J Neurophysiol 91: 1672–1689, 2004 - PubMed
-
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychol (Amst) 112: 105–142, 2003 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

