The progamic phase of an early-divergent angiosperm, Annona cherimola (Annonaceae)
- PMID: 19939980
- PMCID: PMC2814751
- DOI: 10.1093/aob/mcp276
The progamic phase of an early-divergent angiosperm, Annona cherimola (Annonaceae)
Abstract
Background and aims: Recent studies of reproductive biology in ancient angiosperm lineages are beginning to shed light on the early evolution of flowering plants, but comparative studies are restricted by fragmented and meagre species representation in these angiosperm clades. In the present study, the progamic phase, from pollination to fertilization, is characterized in Annona cherimola, which is a member of the Annonaceae, the largest extant family among early-divergent angiosperms. Beside interest due to its phylogenetic position, this species is also an ancient crop with a clear niche for expansion in subtropical climates.
Methods: The kinetics of the reproductive process was established following controlled pollinations and sequential fixation. Gynoecium anatomy, pollen tube pathway, embryo sac and early post-fertilization events were characterized histochemically.
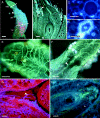
Key results: A plesiomorphic gynoecium with a semi-open carpel shows a continuous secretory papillar surface along the carpel margins, which run from the stigma down to the obturator in the ovary. The pollen grains germinate in the stigma and compete in the stigma-style interface to reach the narrow secretory area that lines the margins of the semi-open stylar canal and is able to host just one to three pollen tubes. The embryo sac has eight nuclei and is well provisioned with large starch grains that are used during early cellular endosperm development.
Conclusions: A plesiomorphic simple gynoecium hosts a simple pollen-pistil interaction, based on a support-control system of pollen tube growth. Support is provided through basipetal secretory activity in the cells that line the pollen tube pathway. Spatial constraints, favouring pollen tube competition, are mediated by a dramatic reduction in the secretory surface available for pollen tube growth at the stigma-style interface. This extramural pollen tube competition contrasts with the intrastylar competition predominant in more recently derived lineages of angiosperms.
Figures





References
-
- APG II. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society. 2003;141:399–436.
-
- Arbeloa A, Herrero M. The significance of the obturator in the control of pollen tube entry into the ovary in peach (Prunus persica) Annals of Botany. 1987;60:681–685.
-
- Arbeloa A, Herrero M. Development of the ovular structures in peach [Prunus-persica (L.) Batsch] New Phytologist. 1991;118:527–533.
-
- Bhandari NN, Venkatar R. Embryology of Drimys winteri. Journal of the Arnold Arboretum. 1968;49:509–525.

