High-throughput pooling and real-time PCR-based strategy for malaria detection
- PMID: 19940051
- PMCID: PMC2815636
- DOI: 10.1128/JCM.01800-09
High-throughput pooling and real-time PCR-based strategy for malaria detection
Abstract
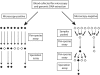
Molecular assays can provide critical information for malaria diagnosis, speciation, and drug resistance, but their cost and resource requirements limit their application to clinical malaria studies. This study describes the application of a resource-conserving testing algorithm employing sample pooling for real-time PCR assays for malaria in a cohort of 182 pregnant women in Kinshasa. A total of 1,268 peripheral blood samples were collected during the study. Using a real-time PCR assay that detects all Plasmodium species, microscopy-positive samples were amplified individually; the microscopy-negative samples were amplified after pooling the genomic DNA (gDNA) of four samples prior to testing. Of 176 microscopy-positive samples, 74 were positive by the real-time PCR assay; the 1,092 microscopy-negative samples were initially amplified in 293 pools, and subsequently, 35 samples were real-time PCR positive (3%). With the real-time PCR result as the referent standard, microscopy was 67.9% sensitive (95% confidence interval [CI], 58.3% to 76.5%) and 91.2% specific (95% CI, 89.4% to 92.8%) for malaria. In total, we detected 109 parasitemias by real-time PCR and, by pooling samples, obviated over 50% of reactions and halved the cost of testing. Our study highlights both substantial discordance between malaria diagnostics and the utility and parsimony of employing a sample pooling strategy for molecular diagnostics in clinical and epidemiologic malaria studies.
Figures

Similar articles
-
PCR-based pooling of dried blood spots for detection of malaria parasites: optimization and application to a cohort of Ugandan children.J Clin Microbiol. 2010 Oct;48(10):3539-43. doi: 10.1128/JCM.00522-10. Epub 2010 Aug 4. J Clin Microbiol. 2010. PMID: 20686079 Free PMC article.
-
Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women.Malar J. 2010 Oct 6;9:269. doi: 10.1186/1475-2875-9-269. Malar J. 2010. PMID: 20925928 Free PMC article.
-
Comparative performance of microscopy, rapid diagnostic tests, and multiplex real-time PCR for detection of malaria parasites among pregnant women in northwest Ethiopia.Malar J. 2025 Jan 20;24(1):19. doi: 10.1186/s12936-025-05256-2. Malar J. 2025. PMID: 39833795 Free PMC article.
-
Pooled PCR testing strategy and prevalence estimation of submicroscopic infections using Bayesian latent class models in pregnant women receiving intermittent preventive treatment at Machinga District Hospital, Malawi, 2010.Malar J. 2014 Dec 18;13:509. doi: 10.1186/1475-2875-13-509. Malar J. 2014. PMID: 25522751 Free PMC article.
-
Effective High-Throughput Blood Pooling Strategy before DNA Extraction for Detection of Malaria in Low-Transmission Settings.Korean J Parasitol. 2016 Jun;54(3):253-9. doi: 10.3347/kjp.2016.54.3.253. Epub 2016 Jun 30. Korean J Parasitol. 2016. PMID: 27417078 Free PMC article.
Cited by
-
Differential contribution of Anopheles coustani and Anopheles arabiensis to the transmission of Plasmodium falciparum and Plasmodium vivax in two neighbouring villages of Madagascar.Parasit Vectors. 2020 Aug 26;13(1):430. doi: 10.1186/s13071-020-04282-0. Parasit Vectors. 2020. PMID: 32843082 Free PMC article.
-
Pooled RNA sample reverse transcriptase real time PCR assay for SARS CoV-2 infection: A reliable, faster and economical method.PLoS One. 2020 Jul 30;15(7):e0236859. doi: 10.1371/journal.pone.0236859. eCollection 2020. PLoS One. 2020. PMID: 32730368 Free PMC article.
-
An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection.Clin Infect Dis. 2013 Jul;57(1):40-7. doi: 10.1093/cid/cit174. Epub 2013 Mar 13. Clin Infect Dis. 2013. PMID: 23487390 Free PMC article.
-
PCR-based pooling of dried blood spots for detection of malaria parasites: optimization and application to a cohort of Ugandan children.J Clin Microbiol. 2010 Oct;48(10):3539-43. doi: 10.1128/JCM.00522-10. Epub 2010 Aug 4. J Clin Microbiol. 2010. PMID: 20686079 Free PMC article.
-
Population, behavioural and environmental drivers of malaria prevalence in the Democratic Republic of Congo.Malar J. 2011 Jun 9;10:161. doi: 10.1186/1475-2875-10-161. Malar J. 2011. PMID: 21658268 Free PMC article.
References
-
- Andrews, L., R. F. Andersen, D. Webster, S. Dunachie, R. M. Walther, P. Bejon, A. Hunt-Cooke, G. Bergson, F. Sanderson, A. V. Hill, and S. C. Gilbert. 2005. Quantitative real-time polymerase chain reaction for malaria diagnosis and its use in malaria vaccine clinical trials. Am. J. Trop. Med. Hyg. 73:191-198. - PubMed
-
- Baliraine, F. N., Y. A. Afrane, D. A. Amenya, M. Bonizzoni, D. M. Menge, G. Zhou, D. Zhong, A. M. Vardo-Zalik, A. K. Githeko, and G. Yan. 2009. High prevalence of asymptomatic Plasmodium falciparum infections in a highland area of western Kenya: a cohort study. J. Infect. Dis. 200:66-74. - PMC - PubMed
-
- Bereczky, S., A. Liljander, I. Rooth, L. Faraja, F. Granath, S. M. Montgomery, and A. Farnert. 2007. Multiclonal asymptomatic Plasmodium falciparum infections predict a reduced risk of malaria disease in a Tanzanian population. Microbes Infect. 9:103-110. - PubMed
-
- Bereczky, S., A. Martensson, J. P. Gil, and A. Farnert. 2005. Short report: rapid DNA extraction from archive blood spots on filter paper for genotyping of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 72:249-251. - PubMed
-
- Bhattarai, A., A. S. Ali, S. P. Kachur, A. Martensson, A. K. Abbas, R. Khatib, A. W. Al-Mafazy, M. Ramsan, G. Rotllant, J. F. Gerstenmaier, F. Molteni, S. Abdulla, S. M. Montgomery, A. Kaneko, and A. Bjorkman. 2007. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 4:e309. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

