Protein-protein interaction between cPLA2 and splice variants of alpha-subunit of BK channels
- PMID: 19940072
- PMCID: PMC3774341
- DOI: 10.1152/ajpcell.00221.2009
Protein-protein interaction between cPLA2 and splice variants of alpha-subunit of BK channels
Abstract
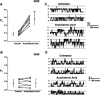
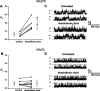
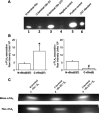
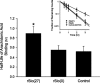
Altering the splice variant composition of large-conductance Ca(2+)-activated potassium (BK) channels can alter their activity and apparent sensitivity to Ca(2+) and other regulators of activity. We hypothesized that differences in the responsiveness to arachidonic acid of GH3 and GH4 cells was due to a difference in two splice variants, one present in GH3 cells and the other in GH4 cells. The sequences of the two splice variants differ from one another in several ways, but the largest difference is the presence or absence of 27 amino acids in the COOH terminus of the BK alpha-subunit. Open probability of the variant containing the 27 amino acids is significantly increased by arachidonic acid, while the variant lacking the 27 amino acids is insensitive to arachidonic acid. In addition, sensitivity of BK channels to arachidonic acid depends on cytosolic phospholipase A(2) (cPLA(2)). Here we used the Mammalian Matchmaker two-hybrid assay and two BK alpha-subunit constructs with [rSlo(27)] and without [rSlo(0)] the 27-amino acid motif to determine whether cPLA(2) associates with one construct [rSlo(27)] and not the other. We hypothesized that differential association of cPLA(2) might explain the differing responsiveness of the two constructs and GH3 and GH4 cells to arachidonic acid. We found that cPLA(2) is strongly associated with the COOH terminus of rSlo(27) and only very weakly associated with rSlo(0). We also found that arachidonic acid has a lower affinity for rSlo(0) than for rSlo(27). We conclude that the lack of response of BK channels in GH4 cells to arachidonic acid can be explained, in part, by the poor binding of cPLA(2) to the COOH terminus of the rSlo(0) alpha-subunit, which is very similar to the splice variant found in the arachidonic acid-insensitive GH4 cells.
Figures






Similar articles
-
Unoprostone activation of BK (KCa1.1) channel splice variants.Biochim Biophys Acta. 2015 Nov;1848(11 Pt A):2859-67. doi: 10.1016/j.bbamem.2015.08.005. Epub 2015 Aug 13. Biochim Biophys Acta. 2015. PMID: 26277265 Free PMC article.
-
Functional effects of auxiliary beta4-subunit on rat large-conductance Ca(2+)-activated K(+) channel.Biophys J. 2004 May;86(5):2871-82. doi: 10.1016/S0006-3495(04)74339-8. Biophys J. 2004. PMID: 15111404 Free PMC article.
-
Splicing of the rSlo gene affects the molecular composition and drug response of Ca2+-activated K+ channels in skeletal muscle.PLoS One. 2012;7(7):e40235. doi: 10.1371/journal.pone.0040235. Epub 2012 Jul 10. PLoS One. 2012. PMID: 22808126 Free PMC article.
-
C2-di-ethyl-ceramide-1-phosphate as an inhibitor of group IVA cytosolic phospholipase A2.Eur J Pharmacol. 2012 Dec 15;697(1-3):144-51. doi: 10.1016/j.ejphar.2012.09.041. Epub 2012 Oct 5. Eur J Pharmacol. 2012. PMID: 23043861
-
Molecular mechanism of pharmacological activation of BK channels.Proc Natl Acad Sci U S A. 2012 Feb 28;109(9):3552-7. doi: 10.1073/pnas.1114321109. Epub 2012 Feb 13. Proc Natl Acad Sci U S A. 2012. PMID: 22331907 Free PMC article.
Cited by
-
BK channels in microglia are required for morphine-induced hyperalgesia.Nat Commun. 2016 May 31;7:11697. doi: 10.1038/ncomms11697. Nat Commun. 2016. PMID: 27241733 Free PMC article.
-
Unoprostone activation of BK (KCa1.1) channel splice variants.Biochim Biophys Acta. 2015 Nov;1848(11 Pt A):2859-67. doi: 10.1016/j.bbamem.2015.08.005. Epub 2015 Aug 13. Biochim Biophys Acta. 2015. PMID: 26277265 Free PMC article.
-
MaxiK channel and cell signalling.Pflugers Arch. 2014 May;466(5):875-86. doi: 10.1007/s00424-013-1359-0. Pflugers Arch. 2014. PMID: 24077696 Free PMC article. Review.
-
Actions and Mechanisms of Polyunsaturated Fatty Acids on Voltage-Gated Ion Channels.Front Physiol. 2017 Feb 6;8:43. doi: 10.3389/fphys.2017.00043. eCollection 2017. Front Physiol. 2017. PMID: 28220076 Free PMC article. Review.
-
Potential tumor-suppressive role of monoglyceride lipase in human colorectal cancer.Oncogene. 2013 Jan 10;32(2):234-41. doi: 10.1038/onc.2012.34. Epub 2012 Feb 20. Oncogene. 2013. PMID: 22349814 Free PMC article.
References
-
- Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000 - PubMed
-
- Denson DD, Li J, Eaton DC. Co-localization of the alpha-subunit of BK-channels and c-PLA2 in GH3 cells. Biochem Biophys Res Commun 350: 39–49, 2006 - PubMed
-
- Denson DD, Wang X, Worrell RT, AlKhalili O, Eaton DC. Cytosolic phospholipase A2 is required for optimal ATP activation of BK channels in GH3 cells. J Biol Chem 276: 7136–7142, 2001 - PubMed
-
- Denson DD, Wang X, Worrell RT, Eaton DC. Effects of fatty acids on BK channels in GH3 cells. Am J Physiol Cell Physiol 279: C1211–C1219, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

