Timing of the initial muscle biopsy does not affect the measured muscle protein fractional synthesis rate during basal, postabsorptive conditions
- PMID: 19940095
- PMCID: PMC2822667
- DOI: 10.1152/japplphysiol.00957.2009
Timing of the initial muscle biopsy does not affect the measured muscle protein fractional synthesis rate during basal, postabsorptive conditions
Abstract
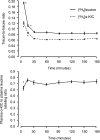
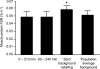
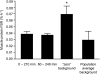
The muscle protein fractional synthesis rate (FSR) is determined by monitoring the incorporation of an amino acid tracer into muscle protein during a constant-rate intravenous tracer infusion. Commonly two sequential muscle biopsies are obtained some time after starting the tracer infusion. However, other protocols, including those with an initial biopsy before starting the tracer infusion to measure the background enrichment and those with only a single biopsy after several hours of tracer infusion have been used. To assess the validity of these approaches, we compared the muscle protein FSR obtained by calculating the difference in [ring-(2)H(5)]phenylalanine and [5,5,5-(2)H(3)]leucine incorporation into muscle protein at approximately 3.5 h after starting the tracer infusion and 1) at 60 min; 2) before starting the tracer infusion (background enrichment); 3) a population average muscle protein background enrichment; and 4) by measuring the tracer incorporation into muscle protein at approximately 3.5 h assuming essentially no background enrichment. Irrespective of the tracer used, the muscle protein FSR calculated from the difference in the muscle protein labeling several hours after starting the tracer infusion and either the labeling at 60 min or the background enrichment were not different (e.g., 0.049 +/- 0.007%/h vs. 0.049 +/- 0.007%/h, respectively, with [(2)H(5)]phenylalanine; P = 0.99). However, omitting the initial biopsy and assuming no background enrichment yielded average FSR values that were approximately 15% (with [(2)H(5)]phenylalanine) to 80% (with [(2)H(3)]leucine) greater (P < or = 0.059); using a population average background enrichment reduced the difference to approximately 3% (P = 0.76) and 22% (P = 0.52) with [(2)H(5)]phenylalanine and [(2)H(3)]leucine, respectively. We conclude that during basal, postabsorptive conditions, valid muscle protein FSR values can be obtained irrespective of the timing of the initial biopsy so long as the protein labeling in two sequential biopsies is measured whereas the single biopsy approach should be avoided.
Figures

Similar articles
-
The single biopsy approach is reliable for the measurement of muscle protein synthesis rates in vivo in older men.J Appl Physiol (1985). 2012 Sep;113(6):896-902. doi: 10.1152/japplphysiol.00513.2012. Epub 2012 Jul 19. J Appl Physiol (1985). 2012. PMID: 22815390
-
Sequential muscle biopsies during a 6-h tracer infusion do not affect human mixed muscle protein synthesis and muscle phenylalanine kinetics.Am J Physiol Endocrinol Metab. 2008 Oct;295(4):E959-63. doi: 10.1152/ajpendo.00671.2007. Epub 2008 Aug 19. Am J Physiol Endocrinol Metab. 2008. PMID: 18713956 Free PMC article.
-
The reliability of using the single-biopsy approach to assess basal muscle protein synthesis rates in vivo in humans.Metabolism. 2012 Jul;61(7):931-6. doi: 10.1016/j.metabol.2011.11.004. Epub 2011 Dec 29. Metabolism. 2012. PMID: 22209666
-
Measurement of human mixed muscle protein fractional synthesis rate depends on the choice of amino acid tracer.Am J Physiol Endocrinol Metab. 2007 Sep;293(3):E666-71. doi: 10.1152/ajpendo.00185.2007. Epub 2007 May 29. Am J Physiol Endocrinol Metab. 2007. PMID: 17535855
-
Human muscle protein turnover--why is it so variable?J Appl Physiol (1985). 2011 Feb;110(2):480-91. doi: 10.1152/japplphysiol.00125.2010. Epub 2010 Nov 25. J Appl Physiol (1985). 2011. PMID: 21109595 Free PMC article. Review.
Cited by
-
β-Adrenergic receptor blockade blunts postexercise skeletal muscle mitochondrial protein synthesis rates in humans.Am J Physiol Regul Integr Comp Physiol. 2011 Aug;301(2):R327-34. doi: 10.1152/ajpregu.00160.2011. Epub 2011 May 25. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21613574 Free PMC article. Clinical Trial.
-
A single bout of resistance exercise improves postprandial lipid metabolism in overweight/obese men with prediabetes.Diabetologia. 2020 Mar;63(3):611-623. doi: 10.1007/s00125-019-05070-x. Epub 2019 Dec 23. Diabetologia. 2020. PMID: 31873788 Free PMC article. Clinical Trial.
-
Influence of tracer selection on protein synthesis rates at rest and postexercise in multiple human muscles.Metabolism. 2011 May;60(5):689-97. doi: 10.1016/j.metabol.2010.07.003. Epub 2010 Sep 6. Metabolism. 2011. PMID: 20822780 Free PMC article.
-
Validation of a single biopsy approach and bolus protein feeding to determine myofibrillar protein synthesis in stable isotope tracer studies in humans.Nutr Metab (Lond). 2011 Mar 9;8:15. doi: 10.1186/1743-7075-8-15. Nutr Metab (Lond). 2011. PMID: 21388545 Free PMC article.
-
Muscle protein synthesis response to exercise training in obese, older men and women.Med Sci Sports Exerc. 2012 Jul;44(7):1259-66. doi: 10.1249/MSS.0b013e3182496a41. Med Sci Sports Exerc. 2012. PMID: 22246218 Free PMC article.
References
-
- Boirie Y, Short KR, Ahlman B, Charlton M, Nair KS. Tissue-specific regulation of mitochondrial and cytoplasmic protein synthesis rates by insulin. Diabetes 50: 2652–2658, 2001 - PubMed
-
- Carroll CC, Fluckey JD, Williams RH, Sullivan DH, Trappe TA. Human soleus and vastus lateralis muscle protein metabolism with an amino acid infusion. Am J Physiol Endocrinol Metab 288: E479–E485, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

