Limiting activity at beta1-subunit-containing GABAA receptor subtypes reduces ataxia
- PMID: 19940102
- PMCID: PMC2835428
- DOI: 10.1124/jpet.109.161885
Limiting activity at beta1-subunit-containing GABAA receptor subtypes reduces ataxia
Abstract
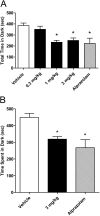
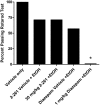
GABA(A) receptor (R) positive allosteric modulators that selectively modulate GABA(A)Rs containing beta(2)- and/or beta(3)- over beta(1)-subunits have been reported across diverse chemotypes. Examples include loreclezole, mefenamic acid, tracazolate, and etifoxine. In general,"beta(2/3)-selective" GABA(A)R positive allosteric modulators are nonbenzodiazepines (nonBZs), do not show alpha-subunit isoform selectivity, yet have anxiolytic efficacy with reduced ataxic/sedative effects in animal models and humans. Here, we report on an enantiomeric pair of nonBZ GABA(A)R positive allosteric modulators that demonstrate differential beta-subunit isoform selectivity. We have tested this enantiomeric pair along with a series of other beta(2/3)-subunit selective, alpha-subunit isoform-selective, BZ and nonBZ GABA(A) positive allosteric modulators using electrophysiological, pharmacokinetic, and behavioral assays to test the hypothesis that ataxia may be correlated with the extent of modulation at beta(1)-subunit-containing GABA(A)Rs. Our findings provide an alternative strategy for designing anxioselective allosteric modulators of the GABA(A)R with BZ-like anxiolytic efficacy by reducing or eliminating activity at beta(1)-subunit-containing GABA(A)Rs.
Figures






Similar articles
-
Flavan-3-ol derivatives are positive modulators of GABA(A) receptors with higher efficacy for the alpha(2) subtype and anxiolytic action in mice.Neuropharmacology. 2008 Oct;55(5):900-7. doi: 10.1016/j.neuropharm.2008.06.069. Epub 2008 Jul 9. Neuropharmacology. 2008. PMID: 18657554
-
The modulatory effects of the anxiolytic etifoxine on GABA(A) receptors are mediated by the beta subunit.Neuropharmacology. 2003 Sep;45(3):293-303. doi: 10.1016/s0028-3908(03)00187-4. Neuropharmacology. 2003. PMID: 12871647
-
The anxioselective agent 7-(2-chloropyridin-4-yl)pyrazolo-[1,5-a]-pyrimidin-3-yl](pyridin-2-yl)methanone (DOV 51892) is more efficacious than diazepam at enhancing GABA-gated currents at alpha1 subunit-containing GABAA receptors.J Pharmacol Exp Ther. 2006 Dec;319(3):1244-52. doi: 10.1124/jpet.106.107201. Epub 2006 Sep 13. J Pharmacol Exp Ther. 2006. PMID: 16971504
-
The GABAA-BZR complex as target for the development of anxiolytic drugs.Curr Top Med Chem. 2012;12(4):254-69. doi: 10.2174/1568026799078787. Curr Top Med Chem. 2012. PMID: 22204488 Review.
-
GABAA receptor subtype-selective modulators. I. α2/α3-selective agonists as non-sedating anxiolytics.Curr Top Med Chem. 2011;11(9):1176-202. doi: 10.2174/156802611795371350. Curr Top Med Chem. 2011. PMID: 21050172 Review.
Cited by
-
Alterations in Purkinje cell GABAA receptor pharmacology following oxygen and glucose deprivation and cerebral ischemia reveal novel contribution of β1 -subunit-containing receptors.Eur J Neurosci. 2013 Feb;37(4):555-63. doi: 10.1111/ejn.12064. Epub 2012 Nov 26. Eur J Neurosci. 2013. PMID: 23176253 Free PMC article.
-
Realising the therapeutic potential of neuroactive steroid modulators of the GABAA receptor.Neurobiol Stress. 2019 Dec 23;12:100207. doi: 10.1016/j.ynstr.2019.100207. eCollection 2020 May. Neurobiol Stress. 2019. PMID: 32435660 Free PMC article.
-
Antiseizure properties of fenamate NSAIDs determined in mature human stem-cell derived neuroglial circuits.Front Pharmacol. 2024 May 16;15:1385523. doi: 10.3389/fphar.2024.1385523. eCollection 2024. Front Pharmacol. 2024. PMID: 38828453 Free PMC article.
-
Use of multicomponent reactions in developing small-molecule tools to study GABAA receptor mechanism and function.Future Med Chem. 2011 Feb;3(2):243-50. doi: 10.4155/fmc.10.302. Future Med Chem. 2011. PMID: 21428818 Free PMC article. Review.
-
α4βδ GABA(A) receptors are high-affinity targets for γ-hydroxybutyric acid (GHB).Proc Natl Acad Sci U S A. 2012 Aug 14;109(33):13404-9. doi: 10.1073/pnas.1204376109. Epub 2012 Jul 2. Proc Natl Acad Sci U S A. 2012. PMID: 22753476 Free PMC article.
References
-
- Asanuma C. (1994) GABAergic and pallidal terminals in the thalamic reticular nucleus of squirrel monkeys. Exp Brain Res 101:439–451 - PubMed
-
- Astleford BA, Goe GL, Keay JG, Scriven EFV. (1989) Synthesis of 1-alkyl-1,2,4-triazoles: a new one-pot regiospecific procedure. J Org Chem 54:731–732
-
- Bare TM, McLaren CD, Campbell JB, Firor JW, Resch JF, Walters CP, Salama AI, Meiners BA, Patel JB. (1989) Synthesis and structure-activity relationships of a series of anxioselective pyrazolopyridine ester and amide anxiolytic agents. J Med Chem 32:2561–2573 - PubMed
-
- Basile AS, Lippa AS, Skolnick P. (2004) Anxioselective anxiolytics: can less be more? Eur J Pharmacol 500:441–451 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

