TILRR, a novel IL-1RI co-receptor, potentiates MyD88 recruitment to control Ras-dependent amplification of NF-kappaB
- PMID: 19940113
- PMCID: PMC2844171
- DOI: 10.1074/jbc.M109.073429
TILRR, a novel IL-1RI co-receptor, potentiates MyD88 recruitment to control Ras-dependent amplification of NF-kappaB
Erratum in
- J Biol Chem. 2010 Jun 4;285(23):18122
Abstract
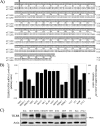
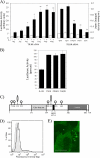
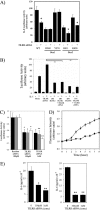
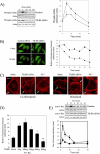
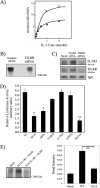
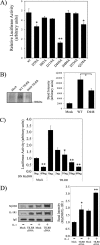
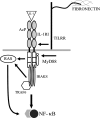
Host defense against infection is induced by Toll-like and interleukin (IL)-1 receptors, and controlled by the transcription factor NF-kappaB. Our earlier studies have shown that IL-1 activation impacts cytoskeletal structure and that IL-1 receptor (IL-1RI) function is substrate-dependent. Here we identify a novel regulatory component, TILRR, which amplifies activation of IL-1RI and coordinates IL-1-induced control with mechanotransduction. We show that TILRR is a highly conserved and widely expressed enhancer of IL-1-regulated inflammatory responses and, further, that it is a membrane-bound glycosylated protein with sequence homology to members of the FRAS-1 family. We demonstrate that TILRR is recruited to the IL-1 receptor complex and magnifies signal amplification by increasing receptor expression and ligand binding. In addition, we show that the consequent potentiation of NF-kappaB is controlled through IL-1RI-associated signaling components in coordination with activation of the Ras GTPase. Using mutagenesis, we demonstrate that TILRR function is dependent on association with its signaling partner and, further, that formation of the TILRR-containing IL-1RI complex imparts enhanced association of the MyD88 adapter during ligand-induced activation of NF-kappaB. We conclude that TILRR is an IL-1RI co-receptor, which associates with the signaling receptor complex to enhance recruitment of MyD88 and control Ras-dependent amplification of NF-kappaB and inflammatory responses.
Figures
Similar articles
-
Distinct control of MyD88 adapter-dependent and Akt kinase-regulated responses by the interleukin (IL)-1RI co-receptor, TILRR.J Biol Chem. 2012 Apr 6;287(15):12348-52. doi: 10.1074/jbc.C111.321711. Epub 2012 Jan 19. J Biol Chem. 2012. PMID: 22262840 Free PMC article.
-
Computational Modelling of NF-κB Activation by IL-1RI and Its Co-Receptor TILRR, Predicts a Role for Cytoskeletal Sequestration of IκBα in Inflammatory Signalling.PLoS One. 2015 Jun 25;10(6):e0129888. doi: 10.1371/journal.pone.0129888. eCollection 2015. PLoS One. 2015. PMID: 26110282 Free PMC article.
-
Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor.Nat Cell Biol. 2000 Jun;2(6):346-51. doi: 10.1038/35014038. Nat Cell Biol. 2000. PMID: 10854325
-
A novel negative regulator for IL-1 receptor and Toll-like receptor 4.Immunol Lett. 2005 Jan 15;96(1):27-31. doi: 10.1016/j.imlet.2004.07.008. Immunol Lett. 2005. PMID: 15585304 Review.
-
TLR-4, IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme.Cell Mol Life Sci. 2008 Oct;65(19):2964-78. doi: 10.1007/s00018-008-8064-8. Cell Mol Life Sci. 2008. PMID: 18535784 Free PMC article. Review.
Cited by
-
Integrated Bioinformatics Identifies FREM1 as a Diagnostic Gene Signature for Heart Failure.Appl Bionics Biomech. 2022 Jun 11;2022:1425032. doi: 10.1155/2022/1425032. eCollection 2022. Appl Bionics Biomech. 2022. Retraction in: Appl Bionics Biomech. 2023 Nov 29;2023:9803720. doi: 10.1155/2023/9803720. PMID: 35726312 Free PMC article. Retracted.
-
The chicken IL-1 family: evolution in the context of the studied vertebrate lineage.Immunogenetics. 2014 Aug;66(7-8):427-38. doi: 10.1007/s00251-014-0780-7. Epub 2014 May 27. Immunogenetics. 2014. PMID: 24863340 Free PMC article. Review.
-
Deficiency of FRAS1-related extracellular matrix 1 (FREM1) causes congenital diaphragmatic hernia in humans and mice.Hum Mol Genet. 2013 Mar 1;22(5):1026-38. doi: 10.1093/hmg/dds507. Epub 2012 Dec 5. Hum Mol Genet. 2013. PMID: 23221805 Free PMC article.
-
A genetic polymorphism of FREM1 is associated with resistance against HIV infection in the Pumwani sex worker cohort.J Virol. 2012 Nov;86(21):11899-905. doi: 10.1128/JVI.01499-12. Epub 2012 Aug 22. J Virol. 2012. PMID: 22915813 Free PMC article.
-
Host genetic susceptibility underlying SARS-CoV-2-associated Multisystem Inflammatory Syndrome in Brazilian Children.Mol Med. 2022 Dec 12;28(1):153. doi: 10.1186/s10020-022-00583-5. Mol Med. 2022. PMID: 36510129 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

