Identification of glycosyltransferase 8 family members as xylosyltransferases acting on O-glucosylated notch epidermal growth factor repeats
- PMID: 19940119
- PMCID: PMC2804315
- DOI: 10.1074/jbc.C109.065409
Identification of glycosyltransferase 8 family members as xylosyltransferases acting on O-glucosylated notch epidermal growth factor repeats
Abstract
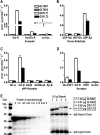
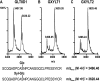
The epidermal growth factor repeats of the Notch receptor are extensively glycosylated with three different O-glycans. O-Fucosylation and elongation by the glycosyltransferase Fringe have been well studied and shown to be essential for proper Notch signaling. In contrast, biosynthesis of O-glucose and O-N-acetylglucosamine is less well understood. Recently, the isolation of the Drosophila mutant rumi has shown that absence of O-glucose impairs Notch function. O-Glucose is further extended by two contiguous alpha1,3-linked xylose residues. We have identified two enzymes of the human glycosyltransferase 8 family, now named GXYLT1 and GXYLT2 (glucoside xylosyltransferase), as UDP-d-xylose:beta-d-glucoside alpha1,3-d-xylosyltransferases adding the first xylose. The enzymes are specific for beta-glucose-terminating acceptors and UDP-xylose as donor substrate. Generation of the alpha1,3-linkage was confirmed by nuclear magnetic resonance. Activity on a natural acceptor could be shown by in vitro xylosylation of a Notch fragment expressed in a UDP-xylose-deficient cell line and in vivo by co-expression of the enzymes and the Notch fragment in insect cells followed by mass spectrometric analysis of peptide fragments.
Figures


References
-
- Moloney D. J., Shair L. H., Lu F. M., Xia J., Locke R., Matta K. L., Haltiwanger R. S. (2000) J. Biol. Chem. 275, 9604–9611 - PubMed
-
- Matsuura A., Ito M., Sakaidani Y., Kondo T., Murakami K., Furukawa K., Nadano D., Matsuda T., Okajima T. (2008) J. Biol. Chem. 283, 35486–35495 - PubMed
-
- Okamura Y., Saga Y. (2008) Mech. Dev. 125, 663–673 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

