Role of Plasmodium berghei cGMP-dependent protein kinase in late liver stage development
- PMID: 19940133
- PMCID: PMC2823412
- DOI: 10.1074/jbc.M109.070367
Role of Plasmodium berghei cGMP-dependent protein kinase in late liver stage development
Abstract
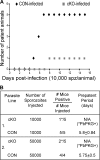
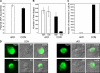
The liver is the first organ infected by Plasmodium sporozoites during malaria infection. In the infected hepatocytes, sporozoites undergo a complex developmental program to eventually generate hepatic merozoites that are released into the bloodstream in membrane-bound vesicles termed merosomes. Parasites blocked at an early developmental stage inside hepatocytes elicit a protective host immune response, making them attractive targets in the effort to develop a pre-erythrocytic stage vaccine. Here, we generated parasites blocked at a late developmental stage inside hepatocytes by conditionally disrupting the Plasmodium berghei cGMP-dependent protein kinase in sporozoites. Mutant sporozoites are able to invade hepatocytes and undergo intracellular development. However, they remain blocked as late liver stages that do not release merosomes into the medium. These late arrested liver stages induce protection in immunized animals. This suggests that, similar to the well studied early liver stages, late stage liver stages too can confer protection from sporozoite challenge.
Figures



Similar articles
-
Overlapping and distinct roles of CDPK family members in the pre-erythrocytic stages of the rodent malaria parasite, Plasmodium berghei.PLoS Pathog. 2020 Aug 31;16(8):e1008131. doi: 10.1371/journal.ppat.1008131. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32866196 Free PMC article.
-
Invasion of hepatocytes by Plasmodium sporozoites requires cGMP-dependent protein kinase and calcium dependent protein kinase 4.Mol Microbiol. 2016 Oct;102(2):349-363. doi: 10.1111/mmi.13466. Epub 2016 Aug 9. Mol Microbiol. 2016. PMID: 27425827 Free PMC article.
-
PKAc is not required for the preerythrocytic stages of Plasmodium berghei.Life Sci Alliance. 2019 May 29;2(3):e201900352. doi: 10.26508/lsa.201900352. Print 2019 Jun. Life Sci Alliance. 2019. PMID: 31142638 Free PMC article.
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
-
Sneaking in through the back entrance: the biology of malaria liver stages.Trends Parasitol. 2004 Sep;20(9):417-24. doi: 10.1016/j.pt.2004.07.007. Trends Parasitol. 2004. PMID: 15324732 Review.
Cited by
-
Overlapping and distinct roles of CDPK family members in the pre-erythrocytic stages of the rodent malaria parasite, Plasmodium berghei.PLoS Pathog. 2020 Aug 31;16(8):e1008131. doi: 10.1371/journal.ppat.1008131. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32866196 Free PMC article.
-
A Plasmodium phospholipase is involved in disruption of the liver stage parasitophorous vacuole membrane.PLoS Pathog. 2015 Mar 18;11(3):e1004760. doi: 10.1371/journal.ppat.1004760. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25786000 Free PMC article.
-
Advances in molecular genetic systems in malaria.Nat Rev Microbiol. 2015 Jun;13(6):373-87. doi: 10.1038/nrmicro3450. Nat Rev Microbiol. 2015. PMID: 25978707 Review.
-
A Plasmodium berghei putative serine-threonine kinase 2 (PBANKA_0311400) is required for late liver stage development and timely initiation of blood stage infection.Biol Open. 2019 Aug 23;8(8):bio042028. doi: 10.1242/bio.042028. Biol Open. 2019. PMID: 31444161 Free PMC article.
-
A toolbox for conditional control of gene expression in apicomplexan parasites.Mol Microbiol. 2022 Mar;117(3):618-631. doi: 10.1111/mmi.14821. Epub 2021 Oct 13. Mol Microbiol. 2022. PMID: 34564906 Free PMC article. Review.
References
-
- Prudêncio M., Rodriguez A., Mota M. M. (2006) Nat. Rev. Microbiol. 4, 849–856 - PubMed
-
- Sturm A., Amino R., van de Sand C., Regen T., Retzlaff S., Rennenberg A., Krueger A., Pollok J. M., Menard R., Heussler V. T. (2006) Science 313, 1287–1290 - PubMed
-
- Mueller A. K., Labaied M., Kappe S. H., Matuschewski K. (2005) Nature 433, 164–167 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

