Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1alpha but Not HIF-2alpha
- PMID: 19940151
- PMCID: PMC2823506
- DOI: 10.1074/jbc.M109.068577
Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1alpha but Not HIF-2alpha
Abstract
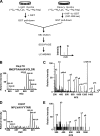
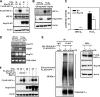
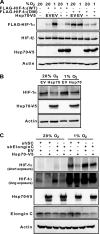
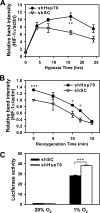
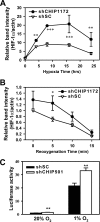
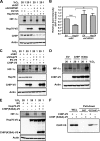
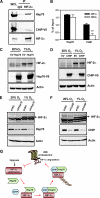
Hypoxia-inducible factors (HIFs) are transcription factors that mediate adaptive responses to reduced oxygen availability. HIF-alpha subunits are stabilized under conditions of acute hypoxia. However, prolonged hypoxia leads to decay of HIF-1alpha but not HIF-2alpha protein levels by unknown mechanisms. Here, we identify Hsp70 and CHIP (carboxyl terminus of Hsc70-interacting protein) as HIF-1alpha-interacting proteins. Hsp70, through recruiting the ubiquitin ligase CHIP, promotes the ubiquitination and proteasomal degradation of HIF-1alpha but not HIF-2alpha, thereby inhibiting HIF-1-dependent gene expression. Disruption of Hsp70-CHIP interaction blocks HIF-1alpha degradation mediated by Hsp70 and CHIP. Inhibition of Hsp70 or CHIP synthesis by RNA interference increases protein levels of HIF-1alpha but not HIF-2alpha and attenuates the decay of HIF-1alpha levels during prolonged hypoxia. Thus, Hsp70- and CHIP-dependent ubiquitination represents a molecular mechanism by which prolonged hypoxia selectively reduces the levels of HIF-1alpha but not HIF-2alpha protein.
Figures




Similar articles
-
The chaperone-dependent ubiquitin ligase CHIP targets HIF-1α for degradation in the presence of methylglyoxal.PLoS One. 2010 Nov 29;5(11):e15062. doi: 10.1371/journal.pone.0015062. PLoS One. 2010. PMID: 21124777 Free PMC article.
-
MYLIP attenuates hypoxia tolerance by inducing K27-linked polyubiquitination and subsequent proteasomal degradation of HIF-α.Commun Biol. 2025 May 21;8(1):774. doi: 10.1038/s42003-025-08200-x. Commun Biol. 2025. PMID: 40399570 Free PMC article.
-
PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1alpha from pVHL-independent degradation.J Biol Chem. 2004 Apr 2;279(14):13506-13. doi: 10.1074/jbc.M310164200. Epub 2004 Jan 15. J Biol Chem. 2004. PMID: 14726529
-
Effects of hypoxia-inducible factor-1α and hypoxia-inducible factor-2α overexpression on hepatocellular carcinoma survival: A systematic review with meta-analysis.J Gastroenterol Hepatol. 2021 Jun;36(6):1487-1496. doi: 10.1111/jgh.15395. Epub 2021 Jan 26. J Gastroenterol Hepatol. 2021. PMID: 33393670
-
HAF : the new player in oxygen-independent HIF-1alpha degradation.Cell Cycle. 2009 May 1;8(9):1359-66. doi: 10.4161/cc.8.9.8303. Cell Cycle. 2009. PMID: 19377289 Free PMC article. Review.
Cited by
-
The role of HIF proteins in maintaining the metabolic health of the intervertebral disc.Nat Rev Rheumatol. 2021 Jul;17(7):426-439. doi: 10.1038/s41584-021-00621-2. Epub 2021 Jun 3. Nat Rev Rheumatol. 2021. PMID: 34083809 Free PMC article. Review.
-
The chaperone-assisted E3 ligase C terminus of Hsc70-interacting protein (CHIP) targets PTEN for proteasomal degradation.J Biol Chem. 2012 May 4;287(19):15996-6006. doi: 10.1074/jbc.M111.321083. Epub 2012 Mar 15. J Biol Chem. 2012. PMID: 22427670 Free PMC article.
-
Bcl-2 regulates HIF-1alpha protein stabilization in hypoxic melanoma cells via the molecular chaperone HSP90.PLoS One. 2010 Jul 27;5(7):e11772. doi: 10.1371/journal.pone.0011772. PLoS One. 2010. Retraction in: PLoS One. 2022 May 3;17(5):e0268235. doi: 10.1371/journal.pone.0268235. PMID: 20668552 Free PMC article. Retracted.
-
Transcriptional regulation by hypoxia inducible factors.Crit Rev Biochem Mol Biol. 2014 Jan-Feb;49(1):1-15. doi: 10.3109/10409238.2013.838205. Epub 2013 Oct 7. Crit Rev Biochem Mol Biol. 2014. PMID: 24099156 Free PMC article. Review.
-
Cyclin-dependent kinases regulate lysosomal degradation of hypoxia-inducible factor 1α to promote cell-cycle progression.Proc Natl Acad Sci U S A. 2014 Aug 12;111(32):E3325-34. doi: 10.1073/pnas.1412840111. Epub 2014 Jul 28. Proc Natl Acad Sci U S A. 2014. PMID: 25071185 Free PMC article.
References
-
- Semenza G. L. (2003) Nat. Rev. Cancer 3, 721–732 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

