Direct observation of individual KCNQ1 potassium channels reveals their distinctive diffusive behavior
- PMID: 19940153
- PMCID: PMC2823507
- DOI: 10.1074/jbc.M109.039974
Direct observation of individual KCNQ1 potassium channels reveals their distinctive diffusive behavior
Abstract
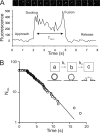
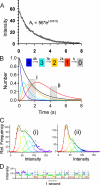
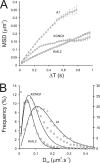
We have directly observed the trafficking and fusion of ion channel containing vesicles and monitored the release of individual ion channels at the plasma membrane of live mammalian cells using total internal reflection fluorescence microscopy. Proteins were fused in-frame with green or red fluorescent proteins and expressed at low level in HL-1 and HEK293 cells. Dual color imaging revealed that vesicle trafficking involved motorized movement along microtubules followed by stalling, fusion, and subsequent release of individual ion channels at the plasma membrane. We found that KCNQ1-KCNE1 complexes were released in batches of about 5 molecules per vesicle. To elucidate the properties of ion channel complexes at the cell membrane we tracked the movement of individual molecules and compared the diffusive behavior of two types of potassium channel complex (KCNQ1-KCNE1 and Kir6.2-SUR2A) to that of a G-protein coupled receptor, the A1 adenosine receptor. Plots of mean squared displacement against time intervals showed that mobility depended on channel type, cell type, and temperature. Analysis of the mobility of wild type KCNQ1-KCNE1 complexes showed the existence of a significant immobile subpopulation and also a significant number of molecules that demonstrated periodic stalling of diffusive movements. This behavior was enhanced in cells treated with jasplakinolide and was abrogated in a C-terminal truncated form (KCNQ1(R518X)-KCNE1) of the protein. This mutant has been identified in patients with the long QT syndrome. We propose that KCNQ1-KCNE1 complexes interact intermittently with the actin cytoskeleton via the C-terminal region and this interaction may have a functional role.
Figures






Similar articles
-
Dynamic partnership between KCNQ1 and KCNE1 and influence on cardiac IKs current amplitude by KCNE2.J Biol Chem. 2009 Jun 12;284(24):16452-16462. doi: 10.1074/jbc.M808262200. Epub 2009 Apr 16. J Biol Chem. 2009. PMID: 19372218 Free PMC article.
-
[Ca2+]i elevation and oxidative stress induce KCNQ1 protein translocation from the cytosol to the cell surface and increase slow delayed rectifier (IKs) in cardiac myocytes.J Biol Chem. 2013 Dec 6;288(49):35358-71. doi: 10.1074/jbc.M113.504746. Epub 2013 Oct 18. J Biol Chem. 2013. PMID: 24142691 Free PMC article.
-
Mechanisms of disease pathogenesis in long QT syndrome type 5.Am J Physiol Cell Physiol. 2010 Feb;298(2):C263-73. doi: 10.1152/ajpcell.00308.2009. Epub 2009 Nov 11. Am J Physiol Cell Physiol. 2010. PMID: 19907016 Free PMC article.
-
Insights into Cardiac IKs (KCNQ1/KCNE1) Channels Regulation.Int J Mol Sci. 2020 Dec 11;21(24):9440. doi: 10.3390/ijms21249440. Int J Mol Sci. 2020. PMID: 33322401 Free PMC article. Review.
-
The membrane protein KCNQ1 potassium ion channel: Functional diversity and current structural insights.Biochim Biophys Acta Biomembr. 2020 May 1;1862(5):183148. doi: 10.1016/j.bbamem.2019.183148. Epub 2019 Dec 9. Biochim Biophys Acta Biomembr. 2020. PMID: 31825788 Free PMC article. Review.
Cited by
-
A method for imaging single molecules at the plasma membrane of live cells within tissue slices.J Gen Physiol. 2021 Jan 4;153(1):e202012657. doi: 10.1085/jgp.202012657. J Gen Physiol. 2021. PMID: 33326014 Free PMC article.
-
TORC2-Gad8-dependent myosin phosphorylation modulates regulation by calcium.Elife. 2019 Sep 30;8:e51150. doi: 10.7554/eLife.51150. Elife. 2019. PMID: 31566560 Free PMC article.
-
Delayed KCNQ1/KCNE1 assembly on the cell surface helps IKs fulfil its function as a repolarization reserve in the heart.J Physiol. 2021 Jul;599(13):3337-3361. doi: 10.1113/JP281773. Epub 2021 Jun 1. J Physiol. 2021. PMID: 33963564 Free PMC article.
-
Resting state Orai1 diffuses as homotetramer in the plasma membrane of live mammalian cells.J Biol Chem. 2010 Dec 24;285(52):41135-42. doi: 10.1074/jbc.M110.177881. Epub 2010 Oct 20. J Biol Chem. 2010. PMID: 20961852 Free PMC article.
-
Partial restoration of the long QT syndrome associated KCNQ1 A341V mutant by the KCNE1 β-subunit.Biochim Biophys Acta. 2011 Dec;1810(12):1285-93. doi: 10.1016/j.bbagen.2011.07.018. Epub 2011 Aug 10. Biochim Biophys Acta. 2011. PMID: 21854832 Free PMC article.
References
-
- Salkoff L., Jegla T. (1995) Neuron 15, 489–492 - PubMed
-
- Barhanin J., Lesage F., Guillemare E., Fink M., Lazdunski M., Romey G. (1996) Nature 384, 78–80 - PubMed
-
- Sanguinetti M. C., Curran M. E., Zou A., Shen J., Spector P. S., Atkinson D. L., Keating M. T. (1996) Nature 384, 80–83 - PubMed
-
- Chen H., Kim L. A., Rajan S., Xu S., Goldstein S. A. (2003) Neuron 40, 15–23 - PubMed
-
- Priori S. G., Barhanin J., Hauer R. N., Haverkamp W., Jongsma H. J., Kleber A. G., McKenna W. J., Roden D. M., Rudy Y., Schwartz K., Schwartz P. J., Towbin J. A., Wilde A. M. (1999) Circulation 99, 518–528 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

