BCAR3 regulates Src/p130 Cas association, Src kinase activity, and breast cancer adhesion signaling
- PMID: 19940159
- PMCID: PMC2807288
- DOI: 10.1074/jbc.M109.046631
BCAR3 regulates Src/p130 Cas association, Src kinase activity, and breast cancer adhesion signaling
Abstract
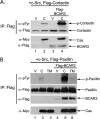
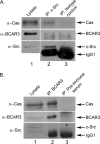
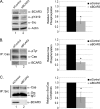
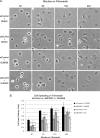
The nonreceptor protein-tyrosine kinase c-Src is frequently overexpressed and/or activated in a variety of cancers, including those of the breast. Several heterologous binding partners of c-Src have been shown to regulate its catalytic activity by relieving intramolecular autoinhibitory interactions. One such protein, p130(Cas) (Cas), is expressed at high levels in both breast cancer cell lines and breast tumors, providing a potential mechanism for c-Src activation in breast cancers. The Cas-binding protein BCAR3 (breast cancer antiestrogen resistance-3) is expressed at high levels in invasive breast cancer cell lines, and this molecule has previously been shown to coordinate with Cas to increase c-Src activity in COS-1 cells. In this study, we show for the first time using gain- and loss-of-function approaches that BCAR3 regulates c-Src activity in the endogenous setting of breast cancer cells. We further show that BCAR3 regulates the interaction between Cas and c-Src, both qualitatively as well as quantitatively. Finally, we present evidence that the coordinated activity of these proteins contributes to breast cancer cell adhesion signaling and spreading. Based on these data, we propose that the c-Src/Cas/BCAR3 signaling axis is a prominent regulator of c-Src activity, which in turn controls cell behaviors that lead to aggressive and invasive breast tumor phenotypes.
Figures


Similar articles
-
Breast cancer anti-estrogen resistance 3 (BCAR3) protein augments binding of the c-Src SH3 domain to Crk-associated substrate (p130cas).J Biol Chem. 2012 Aug 10;287(33):27703-14. doi: 10.1074/jbc.M112.389981. Epub 2012 Jun 18. J Biol Chem. 2012. PMID: 22711540 Free PMC article.
-
Breast cancer antiestrogen resistance 3-p130Cas interactions promote adhesion disassembly and invasion in breast cancer cells.Oncogene. 2016 Nov 10;35(45):5850-5859. doi: 10.1038/onc.2016.123. Epub 2016 Apr 25. Oncogene. 2016. PMID: 27109104 Free PMC article.
-
Synergistic promotion of c-Src activation and cell migration by Cas and AND-34/BCAR3.J Biol Chem. 2003 Jul 25;278(30):28264-73. doi: 10.1074/jbc.M303535200. Epub 2003 May 11. J Biol Chem. 2003. PMID: 12740391
-
Role of SRC-like adaptor protein (SLAP) in immune and malignant cell signaling.Cell Mol Life Sci. 2015 Jul;72(13):2535-44. doi: 10.1007/s00018-015-1882-6. Epub 2015 Mar 13. Cell Mol Life Sci. 2015. PMID: 25772501 Free PMC article. Review.
-
CAS proteins in health and disease: an update.IUBMB Life. 2014 Jun;66(6):387-95. doi: 10.1002/iub.1282. Epub 2014 Jun 24. IUBMB Life. 2014. PMID: 24962474 Free PMC article. Review.
Cited by
-
The SH2 domain protein Shep1 regulates the in vivo signaling function of the scaffolding protein Cas.Cell Signal. 2010 Nov;22(11):1745-52. doi: 10.1016/j.cellsig.2010.06.015. Epub 2010 Jul 24. Cell Signal. 2010. PMID: 20603213 Free PMC article.
-
BCAR3 promotes head and neck cancer growth and is associated with poor prognosis.Cell Death Discov. 2021 Oct 27;7(1):316. doi: 10.1038/s41420-021-00714-7. Cell Death Discov. 2021. PMID: 34707118 Free PMC article.
-
Heparanase promotes human gastric cancer cells migration and invasion by increasing Src and p38 phosphorylation expression.Int J Clin Exp Pathol. 2014 Aug 15;7(9):5609-21. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25337202 Free PMC article.
-
Targeting SRC in mucinous ovarian carcinoma.Clin Cancer Res. 2011 Aug 15;17(16):5367-78. doi: 10.1158/1078-0432.CCR-10-3176. Epub 2011 Jul 7. Clin Cancer Res. 2011. PMID: 21737505 Free PMC article.
-
Deciphering the Role of BCAR3 in Cancer Progression: Gene Regulation, Signal Transduction, and Therapeutic Implications.Cancers (Basel). 2024 Apr 26;16(9):1674. doi: 10.3390/cancers16091674. Cancers (Basel). 2024. PMID: 38730626 Free PMC article. Review.
References
-
- Finn R. S. (2008) Ann. Oncol. 19, 1379–1386 - PubMed
-
- Summy J. M., Gallick G. E. (2003) Cancer Metastasis Rev. 22, 337–358 - PubMed
-
- Gotoh T., Cai D., Tian X., Feig L. A., Lerner A. (2000) J. Biol. Chem. 275, 30118–30123 - PubMed
-
- Lu Y., Brush J., Stewart T. A. (1999) J. Biol. Chem. 274, 10047–10052 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

