Synaptic activity induces dramatic changes in the geometry of the cell nucleus: interplay between nuclear structure, histone H3 phosphorylation, and nuclear calcium signaling
- PMID: 19940164
- PMCID: PMC6666017
- DOI: 10.1523/JNEUROSCI.1160-09.2009
Synaptic activity induces dramatic changes in the geometry of the cell nucleus: interplay between nuclear structure, histone H3 phosphorylation, and nuclear calcium signaling
Abstract
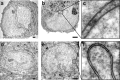
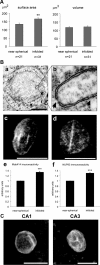
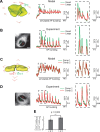
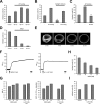
Synaptic activity initiates many adaptive responses in neurons. Here we report a novel form of structural plasticity in dissociated hippocampal cultures and slice preparations. Using a recently developed algorithm for three-dimensional image reconstruction and quantitative measurements of cell organelles, we found that many nuclei from hippocampal neurons are highly infolded and form unequally sized nuclear compartments. Nuclear infoldings are dynamic structures, which can radically transform the geometry of the nucleus in response to neuronal activity. Action potential bursting causing synaptic NMDA receptor activation dramatically increases the number of infolded nuclei via a process that requires the ERK-MAP kinase pathway and new protein synthesis. In contrast, death-signaling pathways triggered by extrasynaptic NMDA receptors cause a rapid loss of nuclear infoldings. Compared with near-spherical nuclei, infolded nuclei have a larger surface and increased nuclear pore complex immunoreactivity. Nuclear calcium signals evoked by cytosolic calcium transients are larger in small nuclear compartments than in the large compartments of the same nucleus; moreover, small compartments are more efficient in temporally resolving calcium signals induced by trains of action potentials in the theta frequency range (5 Hz). Synaptic activity-induced phosphorylation of histone H3 on serine 10 was more robust in neurons with infolded nuclei compared with neurons with near-spherical nuclei, suggesting a functional link between nuclear geometry and transcriptional regulation. The translation of synaptic activity-induced signaling events into changes in nuclear geometry facilitates the relay of calcium signals to the nucleus, may lead to the formation of nuclear signaling microdomains, and could enhance signal-regulated transcription.
Figures




Similar articles
-
A signaling cascade of nuclear calcium-CREB-ATF3 activated by synaptic NMDA receptors defines a gene repression module that protects against extrasynaptic NMDA receptor-induced neuronal cell death and ischemic brain damage.J Neurosci. 2011 Mar 30;31(13):4978-90. doi: 10.1523/JNEUROSCI.2672-10.2011. J Neurosci. 2011. PMID: 21451036 Free PMC article.
-
A quantitative method to assess extrasynaptic NMDA receptor function in the protective effect of synaptic activity against neurotoxicity.BMC Neurosci. 2008 Jan 24;9:11. doi: 10.1186/1471-2202-9-11. BMC Neurosci. 2008. PMID: 18218077 Free PMC article.
-
Synaptic activity and nuclear calcium signaling protect hippocampal neurons from death signal-associated nuclear translocation of FoxO3a induced by extrasynaptic N-methyl-D-aspartate receptors.J Biol Chem. 2010 Jun 18;285(25):19354-61. doi: 10.1074/jbc.M110.127654. Epub 2010 Apr 19. J Biol Chem. 2010. PMID: 20404335 Free PMC article.
-
Activity-dependent calcium signaling and ERK-MAP kinases in neurons: a link to structural plasticity of the nucleus and gene transcription regulation.Cell Calcium. 2011 May;49(5):296-305. doi: 10.1016/j.ceca.2010.11.009. Epub 2010 Dec 15. Cell Calcium. 2011. PMID: 21163523 Review.
-
Nuclear calcium signaling.Adv Exp Med Biol. 2012;970:377-405. doi: 10.1007/978-3-7091-0932-8_17. Adv Exp Med Biol. 2012. PMID: 22351065 Review.
Cited by
-
What Is Required for Neuronal Calcium Waves? A Numerical Parameter Study.J Math Neurosci. 2018 Jul 13;8(1):9. doi: 10.1186/s13408-018-0064-x. J Math Neurosci. 2018. PMID: 30006849 Free PMC article.
-
The nuclear envelope environment and its cancer connections.Nat Rev Cancer. 2012 Feb 16;12(3):196-209. doi: 10.1038/nrc3219. Nat Rev Cancer. 2012. PMID: 22337151 Free PMC article. Review.
-
Intrinsic optical signal imaging of glucose-stimulated insulin secreting β-cells.Opt Express. 2011 Jan 3;19(1):99-106. doi: 10.1364/OE.19.000099. Opt Express. 2011. PMID: 21263546 Free PMC article.
-
Osmotic stress alters chromatin condensation and nucleocytoplasmic transport.Biochem Biophys Res Commun. 2011 May 6;408(2):230-5. doi: 10.1016/j.bbrc.2011.03.131. Epub 2011 Apr 2. Biochem Biophys Res Commun. 2011. PMID: 21463604 Free PMC article.
-
Lamin B1 protein is required for dendrite development in primary mouse cortical neurons.Mol Biol Cell. 2016 Jan 1;27(1):35-47. doi: 10.1091/mbc.E15-05-0307. Epub 2015 Oct 28. Mol Biol Cell. 2016. PMID: 26510501 Free PMC article.
References
-
- Akhtar A, Gasser SM. The nuclear envelope and transcriptional control. Nat Rev Genet. 2007;8:507–517. - PubMed
-
- al-Mohanna FA, Caddy KW, Bolsover SR. The nucleus is insulated from large cytosolic calcium ion changes. Nature. 1994;367:745–750. - PubMed
-
- Augustine GJ, Neher E. Neuronal Ca2+ signalling takes the local route. Curr Opin Neurobiol. 1992;2:302–307. - PubMed
-
- Bading H. Transcription-dependent neuronal plasticity: the nuclear calcium hypothesis. Eur J Biochem. 2000;267:5280–5283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
