Role of striatum in updating values of chosen actions
- PMID: 19940165
- PMCID: PMC6666000
- DOI: 10.1523/JNEUROSCI.2728-09.2009
Role of striatum in updating values of chosen actions
Abstract
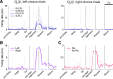
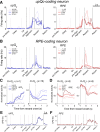
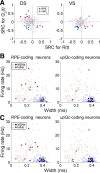
The striatum is thought to play a crucial role in value-based decision making. Although a large body of evidence suggests its involvement in action selection as well as action evaluation, underlying neural processes for these functions of the striatum are largely unknown. To obtain insights on this matter, we simultaneously recorded neuronal activity in the dorsal and ventral striatum of rats performing a dynamic two-armed bandit task, and examined temporal profiles of neural signals related to animal's choice, its outcome, and action value. Whereas significant neural signals for action value were found in both structures before animal's choice of action, signals related to the upcoming choice were relatively weak and began to emerge only in the dorsal striatum approximately 200 ms before the behavioral manifestation of the animal's choice. In contrast, once the animal revealed its choice, signals related to choice and its value increased steeply and persisted until the outcome of animal's choice was revealed, so that some neurons in both structures concurrently conveyed signals related to animal's choice, its outcome, and the value of chosen action. Thus, all the components necessary for updating values of chosen actions were available in the striatum. These results suggest that the striatum not only represents values associated with potential choices before animal's choice of action, but might also update the value of chosen action once its outcome is revealed. In contrast, action selection might take place elsewhere or in the dorsal striatum only immediately before its behavioral manifestation.
Figures


Similar articles
-
Signals for previous goal choice persist in the dorsomedial, but not dorsolateral striatum of rats.J Neurosci. 2013 Jan 2;33(1):52-63. doi: 10.1523/JNEUROSCI.2422-12.2013. J Neurosci. 2013. PMID: 23283321 Free PMC article.
-
Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making.Neuron. 2010 May 13;66(3):449-60. doi: 10.1016/j.neuron.2010.03.033. Neuron. 2010. PMID: 20471357 Free PMC article.
-
Encoding of action history in the rat ventral striatum.J Neurophysiol. 2007 Dec;98(6):3548-56. doi: 10.1152/jn.00310.2007. Epub 2007 Oct 17. J Neurophysiol. 2007. PMID: 17942629
-
Striatal contributions to reward and decision making: making sense of regional variations in a reiterated processing matrix.Ann N Y Acad Sci. 2007 May;1104:192-212. doi: 10.1196/annals.1390.016. Epub 2007 Apr 7. Ann N Y Acad Sci. 2007. PMID: 17416920 Review.
-
The integrative function of the basal ganglia in instrumental conditioning.Behav Brain Res. 2009 Apr 12;199(1):43-52. doi: 10.1016/j.bbr.2008.10.034. Epub 2008 Nov 5. Behav Brain Res. 2009. PMID: 19027797 Review.
Cited by
-
It's a pleasure: a tale of two cortical areas.Nat Neurosci. 2011 Nov 23;14(12):1491-2. doi: 10.1038/nn.2981. Nat Neurosci. 2011. PMID: 22119944 No abstract available.
-
Meta-reinforcement learning via orbitofrontal cortex.Nat Neurosci. 2023 Dec;26(12):2182-2191. doi: 10.1038/s41593-023-01485-3. Epub 2023 Nov 13. Nat Neurosci. 2023. PMID: 37957318 Free PMC article.
-
Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value.Nat Neurosci. 2012 Sep;15(9):1281-9. doi: 10.1038/nn.3188. Epub 2012 Aug 19. Nat Neurosci. 2012. PMID: 22902719 Free PMC article.
-
Signals for previous goal choice persist in the dorsomedial, but not dorsolateral striatum of rats.J Neurosci. 2013 Jan 2;33(1):52-63. doi: 10.1523/JNEUROSCI.2422-12.2013. J Neurosci. 2013. PMID: 23283321 Free PMC article.
-
Activation, but not inhibition, of the indirect pathway disrupts choice rejection in a freely moving, multiple-choice foraging task.Cell Rep. 2022 Jul 26;40(4):111129. doi: 10.1016/j.celrep.2022.111129. Cell Rep. 2022. PMID: 35905722 Free PMC article.
References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990a;13:266–271. - PubMed
-
- Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990b;64:133–150. - PubMed
-
- Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol. 1992;68:945–960. - PubMed
-
- Atallah HE, Lopez-Paniagua D, Rudy JW, O'Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nat Neurosci. 2007;10:126–131. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
