Tripartite purinergic modulation of central respiratory networks during perinatal development: the influence of ATP, ectonucleotidases, and ATP metabolites
- PMID: 19940166
- PMCID: PMC6666021
- DOI: 10.1523/JNEUROSCI.2660-09.2009
Tripartite purinergic modulation of central respiratory networks during perinatal development: the influence of ATP, ectonucleotidases, and ATP metabolites
Abstract
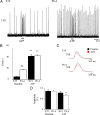
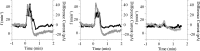
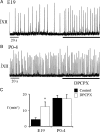
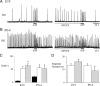
ATP released during hypoxia from the ventrolateral medulla activates purinergic receptors (P2Rs) to attenuate the secondary hypoxic depression of breathing by a mechanism that likely involves a P2Y(1)R-mediated excitation of preBötzinger complex (preBötC) inspiratory rhythm-generating networks. In this study, we used rhythmically active in vitro preparations from embryonic and postnatal rats and ATP microinjection into the rostral ventral respiratory group (rVRG)/preBötC to reveal that these networks are sensitive to ATP when rhythm emerges at embryonic day 17 (E17). The peak frequency elicited by ATP at E19 and postnatally was the same ( approximately 45 bursts/min), but relative sensitivity was threefold greater at E19, reflecting a lower baseline frequency (5.6 +/- 0.9 vs 19.0 +/- 1.3 bursts/min). Combining microinjection techniques with ATP biosensors revealed that ATP concentration in the rVRG/preBötC falls rapidly as a result of active processes and closely correlates with inspiratory frequency. A phosphate assay established that preBötC-containing tissue punches degrade ATP at rates that increase perinatally. Thus, the agonist profile [ATP/ADP/adenosine (ADO)] produced after ATP release in the rVRG/preBötC will change perinatally. Electrophysiology further established that the ATP metabolite ADP is excitatory and that, in fetal but not postnatal animals, ADO at A(1) receptors exerts a tonic depressive action on rhythm, whereas A(1) antagonists extend the excitatory action of ATP on inspiratory rhythm. These data demonstrate that ATP is a potent excitatory modulator of the rVRG/preBötC inspiratory network from the time it becomes active and that ATP actions are determined by a dynamic interaction between the actions of ATP at P2 receptors, ectonucleotidases that degrade ATP, and ATP metabolites on P2Y and P1 receptors.
Figures





Similar articles
-
Purinergic modulation of preBötzinger complex inspiratory rhythm in rodents: the interaction between ATP and adenosine.J Physiol. 2011 Sep 15;589(Pt 18):4583-600. doi: 10.1113/jphysiol.2011.210930. Epub 2011 Jul 25. J Physiol. 2011. PMID: 21788352 Free PMC article.
-
Cholinergic neurotransmission in the preBötzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm.Neuroscience. 2005;130(4):1069-81. doi: 10.1016/j.neuroscience.2004.10.028. Neuroscience. 2005. PMID: 15653001 Free PMC article.
-
P2Y1 receptor modulation of the pre-Bötzinger complex inspiratory rhythm generating network in vitro.J Neurosci. 2007 Jan 31;27(5):993-1005. doi: 10.1523/JNEUROSCI.3948-06.2007. J Neurosci. 2007. PMID: 17267553 Free PMC article.
-
Neuromodulation: purinergic signaling in respiratory control.Compr Physiol. 2013 Jan;3(1):331-63. doi: 10.1002/cphy.c120004. Compr Physiol. 2013. PMID: 23720290 Review.
-
Purinergic signaling in the modulation of redox biology.Redox Biol. 2021 Nov;47:102137. doi: 10.1016/j.redox.2021.102137. Epub 2021 Sep 22. Redox Biol. 2021. PMID: 34563872 Free PMC article. Review.
Cited by
-
Regulation of adenosine levels during cerebral ischemia.Acta Pharmacol Sin. 2013 Jan;34(1):60-6. doi: 10.1038/aps.2012.127. Epub 2012 Oct 15. Acta Pharmacol Sin. 2013. PMID: 23064722 Free PMC article. Review.
-
Functional Oxygen Sensitivity of Astrocytes.J Neurosci. 2015 Jul 22;35(29):10460-73. doi: 10.1523/JNEUROSCI.0045-15.2015. J Neurosci. 2015. PMID: 26203141 Free PMC article.
-
Morphometric analysis of astrocytes in brainstem respiratory regions.J Comp Neurol. 2018 Sep 1;526(13):2032-2047. doi: 10.1002/cne.24472. Epub 2018 Aug 22. J Comp Neurol. 2018. PMID: 29888789 Free PMC article.
-
P2Y1 receptor-mediated potentiation of inspiratory motor output in neonatal rat in vitro.J Physiol. 2014 Jul 15;592(14):3089-111. doi: 10.1113/jphysiol.2013.268136. Epub 2014 May 30. J Physiol. 2014. PMID: 24879869 Free PMC article.
-
ATP, glia and central respiratory control.Respir Physiol Neurobiol. 2010 Oct 31;173(3):305-11. doi: 10.1016/j.resp.2010.06.009. Epub 2010 Jun 23. Respir Physiol Neurobiol. 2010. PMID: 20601205 Free PMC article. Review.
References
-
- Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–55. - PMC - PubMed
-
- Alvarado-Castillo C, Harden TK, Boyer JL. Regulation of P2Y1 receptor-mediated signaling by the ectonucleoside triphosphate diphosphohydrolase isozymes NTPDase1 and NTPDase2. Mol Pharmacol. 2005;67:114–122. - PubMed
-
- Anguio y Gonzalez. The prenatal growth of the albino rat. Anat Rec. 1932;52:117–138.
-
- Barrington K, Finer N. The natural history of the appearance of apnea of prematurity. Pediatr Res. 1991;29:372–375. - PubMed
-
- Bhatt-Mehta V, Schumacher RE. Treatment of apnea of prematurity. Paediatr Drugs. 2003;5:195–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
