Proneurotrophin-3 is a neuronal apoptotic ligand: evidence for retrograde-directed cell killing
- PMID: 19940174
- PMCID: PMC2824605
- DOI: 10.1523/JNEUROSCI.2059-09.2009
Proneurotrophin-3 is a neuronal apoptotic ligand: evidence for retrograde-directed cell killing
Abstract
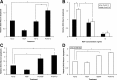
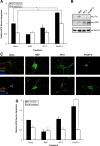
Although mature neurotrophins are well described trophic factors that elicit retrograde survival signaling, the precursor forms of neurotrophins (i.e., proneurotrophins) can function as high-affinity apoptotic ligands for selected neural populations. An outstanding question is whether target-derived proneurotrophins might affect neuronal survival/death decisions through a retrograde transport mechanism. Since neurotrophin-3 (NT-3) is highly expressed in non-neural tissues that receive peripheral innervation, we investigated the localized actions of its precursor (proNT-3) on sympathetic neurons in the present study. Pharmacological inhibition of intracellular furin proteinase activity in 293T cells resulted in proNT-3 release instead of mature NT-3, whereas membrane depolarization in cerebellar granule neurons stimulated endogenous proNT-3 secretion, suggesting that proNT-3 is an inducible bona fide ligand in the nervous system. Our data also indicate that recombinant proNT-3 induced sympathetic neuron death that is p75(NTR)- and sortilin-dependent, with hallmark features of apoptosis including JNK (c-Jun N-terminal kinase) activation and nuclear fragmentation. Using compartmentalized culture systems that segregate neuronal cell bodies from axons, proNT-3, acting within the distal axon compartment, elicited sympathetic neuron death and overrode the survival-promoting actions of NGF. Together, these results raise the intriguing possibility that dysregulation of proneurotrophin processing/release by innervated targets can be deleterious to the neurons projecting to these sites.
Figures






Similar articles
-
Proneurotrophin-3 may induce Sortilin-dependent death in inner ear neurons.Eur J Neurosci. 2011 Feb;33(4):622-31. doi: 10.1111/j.1460-9568.2010.07556.x. Epub 2011 Jan 24. Eur J Neurosci. 2011. PMID: 21261755 Free PMC article.
-
A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling.Cell. 2004 Jul 23;118(2):243-55. doi: 10.1016/j.cell.2004.06.021. Cell. 2004. PMID: 15260993
-
Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins.J Neurosci. 2006 Jul 19;26(29):7756-66. doi: 10.1523/JNEUROSCI.1560-06.2006. J Neurosci. 2006. PMID: 16855103 Free PMC article.
-
Peptides other than the neurotrophins that can be cleaved from proneurotrophins: a neglected story.Arch Physiol Biochem. 2007 Oct-Dec;113(4-5):228-33. doi: 10.1080/13813450701531250. Arch Physiol Biochem. 2007. PMID: 17917853 Review.
-
Proteolytic processing of the p75 neurotrophin receptor: A prerequisite for signalling?: Neuronal life, growth and death signalling are crucially regulated by intra-membrane proteolysis and trafficking of p75(NTR).Bioessays. 2011 Aug;33(8):614-25. doi: 10.1002/bies.201100036. Epub 2011 Jun 30. Bioessays. 2011. PMID: 21717487 Review.
Cited by
-
Retrograde Degenerative Signaling Mediated by the p75 Neurotrophin Receptor Requires p150Glued Deacetylation by Axonal HDAC1.Dev Cell. 2018 Aug 6;46(3):376-387.e7. doi: 10.1016/j.devcel.2018.07.001. Dev Cell. 2018. PMID: 30086304 Free PMC article.
-
Proneurotrophin-3 may induce Sortilin-dependent death in inner ear neurons.Eur J Neurosci. 2011 Feb;33(4):622-31. doi: 10.1111/j.1460-9568.2010.07556.x. Epub 2011 Jan 24. Eur J Neurosci. 2011. PMID: 21261755 Free PMC article.
-
Degeneration keeps axons on the straight and narrow.Nat Neurosci. 2010 May;13(5):526-8. doi: 10.1038/nn0510-526. Nat Neurosci. 2010. PMID: 20421896 No abstract available.
-
Decellularized peripheral nerve as an injectable delivery vehicle for neural applications.J Biomed Mater Res A. 2022 Mar;110(3):595-611. doi: 10.1002/jbm.a.37312. Epub 2021 Sep 29. J Biomed Mater Res A. 2022. PMID: 34590403 Free PMC article.
-
Maternal Hyperhomocysteinemia Disturbs the Mechanisms of Embryonic Brain Development and Its Maturation in Early Postnatal Ontogenesis.Cells. 2023 Jan 3;12(1):189. doi: 10.3390/cells12010189. Cells. 2023. PMID: 36611982 Free PMC article.
References
-
- Al-Shawi R, Hafner A, Olsen J, Olson J, Chun S, Raza S, Thrasivoulou C, Lovestone S, Killick R, Simons P, Cowen T. Neurotoxic and neurotrophic roles of proNGF and the receptor sortilin in the adult and ageing nervous system. Eur J Neurosci. 2008;27:2103–2114. - PubMed
-
- Althaus HH, Klöppner S. Mature pig oligodendrocytes rapidly process human recombinant pro-nerve growth factor and do not undergo cell death. J Neurochem. 2006;98:506–517. - PubMed
-
- Bates B, Rios M, Trumpp A, Chen C, Fan G, Bishop JM, Jaenisch R. Neurotrophin-3 is required for proper cerebellar development. Nat Neurosci. 1999;2:115–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
