Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons
- PMID: 19940179
- PMCID: PMC6665996
- DOI: 10.1523/JNEUROSCI.2623-09.2009
Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons
Abstract
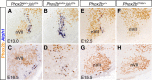
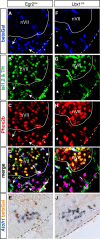
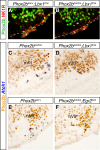
The retrotrapezoid nucleus (RTN) is a group of neurons in the rostral medulla, defined here as Phox2b-, Vglut2-, neurokinin1 receptor-, and Atoh1-expressing cells in the parafacial region, which have been proposed to function both as generators of respiratory rhythm and as central respiratory chemoreceptors. The present study was undertaken to assess these two putative functions using genetic tools. We generated two conditional Phox2b mutations, which target different subsets of Phox2b-expressing cells, but have in common a massive depletion of RTN neurons. In both conditional mutants as well as in the previously described Phox2b(27Ala) mutants, in which the RTN is also compromised, the respiratory-like rhythmic activity normally seen in the parafacial region of fetal brainstem preparations was completely abrogated. Rhythmic motor bursts were recorded from the phrenic nerve roots in the mutants, but their frequency was markedly reduced. Both the rhythmic activity in the RTN region and the phrenic nerve discharges responded to a low pH challenge in control, but not in the mutant embryos. Together, our results provide genetic evidence for the essential role of the Phox2b-expressing RTN neurons both in establishing a normal respiratory rhythm before birth and in providing chemosensory drive.
Figures




References
-
- Akazawa C, Ishibashi M, Shimizu C, Nakanishi S, Kageyama R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem. 1995;270:8730–8738. - PubMed
-
- Amiel J, Laudier B, Attié-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frame shift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. - PubMed
-
- Amiel J, Dubreuil V, Ramanantsoa N, Fortin G, Gallego J, Brunet J-F, Goridis C. PHOX2B in respiratory control: lessons from congenital central hypoventilation syndrome and its mouse models. Respir Physiol Neurobiol. 2009;168:125–132. - PubMed
-
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, Bellen HJ, Fritzsch B, Zoghbi HY. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30:411–422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
