Switching retinogeniculate axon laterality leads to normal targeting but abnormal eye-specific segregation that is activity dependent
- PMID: 19940181
- PMCID: PMC2829946
- DOI: 10.1523/JNEUROSCI.3462-09.2009
Switching retinogeniculate axon laterality leads to normal targeting but abnormal eye-specific segregation that is activity dependent
Abstract
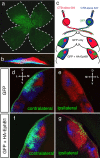
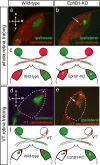
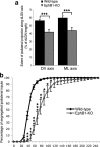
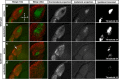
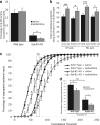
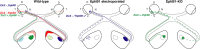
Partial decussation of sensory pathways allows neural inputs from both sides of the body to project to the same target region where these signals will be integrated. Here, to better understand mechanisms of eye-specific targeting, we studied how retinal ganglion cell (RGC) axons terminate in their thalamic target, the dorsal lateral geniculate nucleus (dLGN), when crossing at the optic chiasm midline is altered. In models with gain- and loss-of-function of EphB1, the receptor that directs the ipsilateral projection at the optic chiasm, misrouted RGCs target the appropriate retinotopic zone in the opposite dLGN. However, in EphB1(-/-) mice, the misrouted axons do not intermingle with normally projecting RGC axons and segregate instead into a distinct patch. We also revisited the role of retinal activity on eye-specific targeting by blocking correlated waves of activity with epibatidine into both eyes. We show that, in wild-type mice, retinal waves are necessary during the first postnatal week for both proper distribution and eye-specific segregation of ipsilateral axons in the mature dLGN. Moreover, in EphB1(-/-) mice, refinement of ipsilateral axons is perturbed in control conditions and is further impaired after epibatidine treatment. Finally, retinal waves are required for the formation of the segregated patch of misrouted axons in EphB1(-/-) mice. These findings implicate molecular determinants for targeting of eye-specific zones that are independent of midline guidance cues and that function in concert with correlated retinal activity to sculpt retinogeniculate projections.
Figures

References
-
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. - PMC - PubMed
-
- Beg AA, Sommer JE, Martin JH, Scheiffele P. alpha2-Chimaerin is an essential EphA4 effector in the assembly of neuronal locomotor circuits. Neuron. 2007;55:768–778. - PubMed
-
- Bjartmar L, Huberman AD, Ullian EM, Rentería RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, Cho R, Worley P, Malenka RC, Ball S, Peachey NS, Copenhagen D, Chapman B, Nakamoto M, Barres BA, Perin MS. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–6281. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
