The dorsomedial striatum reflects response bias during learning
- PMID: 19940185
- PMCID: PMC6666004
- DOI: 10.1523/JNEUROSCI.4060-09.2009
The dorsomedial striatum reflects response bias during learning
Abstract
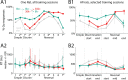
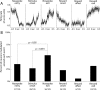
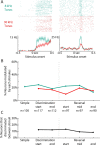
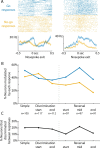
Previous studies have established that neurons in the dorsomedial striatum track the behavioral significance of external stimuli, are sensitive to contingencies between actions and outcomes, and show rapid flexibility in representing task-related information. Here, we describe how neural activity in the dorsomedial striatum changes during the initial acquisition of a Go/NoGo task and during an initial reversal of stimulus-response contingencies. Rats made nosepoke responses over delay periods and then received one of two acoustic stimuli. Liquid rewards were delivered after one stimulus (S+) if the rats made a Go response (entering a reward port on the opposite wall of the chamber). If a Go response was made to other stimulus (S-), rats experienced a timeout. On 10% of trials, no stimulus was presented. These trials were used to assess response bias, the animals' tendency to collect reward independent of the stimulus. Response bias increased during the reversal, corresponding to the animals' uncertainty about the stimulus-response contingencies. Most task-modulated neurons fired during the response at the end of the delay period. The fraction of response-modulated neurons was correlated with response bias and neural activity was sensitive to the behavioral response made on the previous trial. During initial task acquisition and initial reversal learning, there was a remarkable change in the percentages of neurons that fired in relation to the task events, especially during withdrawal from the nosepoke aperture. These results suggest that changes in task-related activity in the dorsomedial striatum during learning are driven by the animal's bias to collect rewards.
Figures



Similar articles
-
Role of striatum in updating values of chosen actions.J Neurosci. 2009 Nov 25;29(47):14701-12. doi: 10.1523/JNEUROSCI.2728-09.2009. J Neurosci. 2009. PMID: 19940165 Free PMC article.
-
Neuronal correlates of instrumental learning in the dorsal striatum.J Neurophysiol. 2009 Jul;102(1):475-89. doi: 10.1152/jn.00262.2009. Epub 2009 May 13. J Neurophysiol. 2009. PMID: 19439679 Free PMC article.
-
Pavlovian influences on learning differ between rats and mice in a counter-balanced Go/NoGo judgement bias task.Behav Brain Res. 2017 Jul 28;331:214-224. doi: 10.1016/j.bbr.2017.05.044. Epub 2017 May 23. Behav Brain Res. 2017. PMID: 28549647 Free PMC article.
-
Single unit activity during a Go/NoGo task in the "prefrontal cortex" of pigeons.Brain Res. 1999 Aug 28;839(2):263-78. doi: 10.1016/s0006-8993(99)01727-8. Brain Res. 1999. PMID: 10519049
-
Dynamic encoding of action selection by the medial striatum.J Neurosci. 2009 Mar 11;29(10):3148-59. doi: 10.1523/JNEUROSCI.5206-08.2009. J Neurosci. 2009. PMID: 19279252 Free PMC article.
Cited by
-
Significance of input correlations in striatal function.PLoS Comput Biol. 2011 Nov;7(11):e1002254. doi: 10.1371/journal.pcbi.1002254. Epub 2011 Nov 17. PLoS Comput Biol. 2011. PMID: 22125480 Free PMC article.
-
Temporal Learning Among Prefrontal and Striatal Ensembles.Cereb Cortex Commun. 2020 Aug 29;1(1):tgaa058. doi: 10.1093/texcom/tgaa058. eCollection 2020. Cereb Cortex Commun. 2020. PMID: 34296121 Free PMC article.
-
Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum.Eur J Neurosci. 2011 Dec;34(12):1997-2006. doi: 10.1111/j.1460-9568.2011.07914.x. Epub 2011 Nov 29. Eur J Neurosci. 2011. PMID: 22122410 Free PMC article.
-
Multiplicity of control in the basal ganglia: computational roles of striatal subregions.Curr Opin Neurobiol. 2011 Jun;21(3):374-80. doi: 10.1016/j.conb.2011.02.009. Epub 2011 Mar 21. Curr Opin Neurobiol. 2011. PMID: 21429734 Free PMC article. Review.
-
Experience-related enhancements in striatal temporal encoding.Eur J Neurosci. 2021 Aug;54(3):5063-5074. doi: 10.1111/ejn.15344. Epub 2021 Jun 29. Eur J Neurosci. 2021. PMID: 34097793 Free PMC article.
References
-
- Adams S, Kesner RP, Ragozzino ME. Role of the medial and lateral caudate-putamen in mediating an auditory conditional response association. Neurobiol Learn Mem. 2001;76:106–116. - PubMed
-
- Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res. 1991;85:491–500. - PubMed
-
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
