Distinct muscarinic acetylcholine receptor subtypes contribute to stability and growth, but not compensatory plasticity, of neuromuscular synapses
- PMID: 19940190
- PMCID: PMC2822401
- DOI: 10.1523/JNEUROSCI.2276-09.2009
Distinct muscarinic acetylcholine receptor subtypes contribute to stability and growth, but not compensatory plasticity, of neuromuscular synapses
Abstract
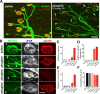
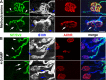
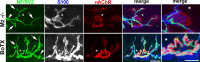
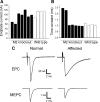
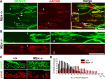
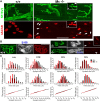
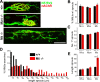
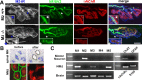
Muscarinic acetylcholine receptors (mAChRs) modulate synaptic function, but whether they influence synaptic structure remains unknown. At neuromuscular junctions (NMJs), mAChRs have been implicated in compensatory sprouting of axon terminals in paralyzed or denervated muscles. Here we used pharmacological and genetic inhibition and localization studies of mAChR subtypes at mouse NMJs to demonstrate their roles in synaptic stability and growth but not in compensatory sprouting. M(2) mAChRs were present solely in motor neurons, whereas M(1), M(3), and M(5) mAChRs were associated with Schwann cells and/or muscle fibers. Blockade of all five mAChR subtypes with atropine evoked pronounced effects, including terminal sprouting, terminal withdrawal, and muscle fiber atrophy. In contrast, methoctramine, an M(2/4)-preferring antagonist, induced terminal sprouting and terminal withdrawal, but no muscle fiber atrophy. Consistent with this observation, M(2)(-/-) but no other mAChR mutant mice exhibited spontaneous sprouting accompanied by extensive loss of parental terminal arbors. Terminal sprouting, however, seemed not to be the causative defect because partial loss of terminal branches was common even in the M(2)(-/-) NMJs without sprouting. Moreover, compensatory sprouting after paralysis or partial denervation was normal in mice deficient in M(2) or other mAChR subtypes. We also found that many NMJs of M(5)(-/-) mice were exceptionally small and reduced in proportion to the size of parental muscle fibers. These findings show that axon terminals are unstable without M(2) and that muscle fiber growth is defective without M(5). Subtype-specific muscarinic signaling provides a novel means for coordinating activity-dependent development and maintenance of the tripartite synapse.
Figures

References
-
- Albani M, Lowrie MB, Vrbová G. Reorganization of motor units in reinnervated muscles of the rat. J Neurol Sci. 1988;88:195–206. - PubMed
-
- Angaut-Petit D, Molgó J, Faille L, Juzans P, Takahashi M. Incorporation of synaptotagmin II to the axolemma of botulinum type-A poisoned mouse motor endings during enhanced quantal acetylcholine release. Brain Res. 1998;797:357–360. - PubMed
-
- Argentieri TM, Aiken SP, Laxminarayan S, McArdle JJ. Characteristics of synaptic transmission in reinnervating rat skeletal muscle. Pflugers Arch. 1992;421:256–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
