Cleavage of proBDNF to BDNF by a tolloid-like metalloproteinase is required for acquisition of in vitro eyeblink classical conditioning
- PMID: 19940191
- PMCID: PMC2825740
- DOI: 10.1523/JNEUROSCI.3649-09.2009
Cleavage of proBDNF to BDNF by a tolloid-like metalloproteinase is required for acquisition of in vitro eyeblink classical conditioning
Abstract
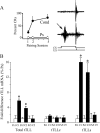
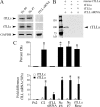
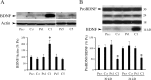
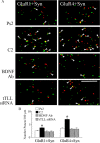
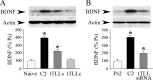
The tolloid/bone morphogenetic protein-1 family of metalloproteinases have an important role in the regulation of embryonic pattern formation and tissue morphogenesis. Studies suggest that they participate in mechanisms of synaptic plasticity in adults, but very little is known about their function. Recently, we isolated a reptilian ortholog of the tolloid gene family designated turtle tolloid-like gene (tTll). Here, we examined the role of tTLL in an in vitro model of eyeblink classical conditioning using an isolated brainstem preparation to assess its role in synaptic plasticity during conditioning. Analysis by real-time reverse transcription-PCR shows that an extracellularly secreted form of tTLL, tTLLs, is transiently expressed in the early stages of conditioning during conditioned response acquisition, whereas a cytosolic form, tTLLc, is not. Short interfering RNA (siRNA)-directed gene knockdown and rescue of tTLL expression demonstrate that it is required for conditioning. Significantly, we show that tTLLs cleaves the precursor proBDNF into mature BDNF in cleavage assay studies, and application of recombinant tTLLs protein alone to preparations results in induction of mature BDNF expression. The mature form of BDNF is minimally expressed in preparations treated with anti-tTLL siRNA, and the synaptic incorporation of both GluR1- and GluR4-containing AMPA receptors is significantly reduced, resulting in suppression of conditioning. This is the first study to demonstrate that expression of an extracellularly secreted tolloid-like metalloproteinase is regulated in the early stages of classical conditioning and functions in the conversion of proBDNF to mature BDNF. The mature form of BDNF is required for synaptic delivery of AMPA receptors and acquisition of conditioned responses.
Figures

References
-
- Anderson CW, Keifer J. Properties of conditioned abducens nerve responses in a highly reduced in vitro brainstem preparation from the turtle. J Neurophysiol. 1999;81:1242–1250. - PubMed
-
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. - PubMed
-
- Bode W, Gomis-Rüth FX, Huber R, Zwilling R, Stöcker W. Structure of astacin and implications for activation of astacins and zinc-ligation of collagenases. Nature. 1992;358:164–167. - PubMed
-
- Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation, and biological functions. Eur J Cell Biol. 1997;74:111–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
