Inhibition of adult rat retinal ganglion cells by D1-type dopamine receptor activation
- PMID: 19940196
- PMCID: PMC3236800
- DOI: 10.1523/JNEUROSCI.3827-09.2009
Inhibition of adult rat retinal ganglion cells by D1-type dopamine receptor activation
Abstract
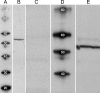
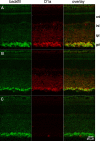
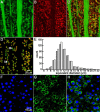
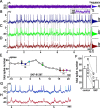
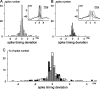
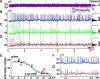
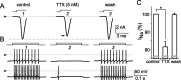
The spike output of neural pathways can be regulated by modulating output neuron excitability and/or their synaptic inputs. Dopaminergic interneurons synapse onto cells that route signals to mammalian retinal ganglion cells, but it is unknown whether dopamine can activate receptors in these ganglion cells and, if it does, how this affects their excitability. Here, we show D(1a) receptor-like immunoreactivity in ganglion cells identified in adult rats by retrogradely transported dextran, and that dopamine, D(1)-type receptor agonists, and cAMP analogs inhibit spiking in ganglion cells dissociated from adult rats. These ligands curtailed repetitive spiking during constant current injections and reduced the number and rate of rise of spikes elicited by fluctuating current injections without significantly altering the timing of the remaining spikes. Consistent with mediation by D(1)-type receptors, SCH-23390 [R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine] reversed the effects of dopamine on spikes. Contrary to a recent report, spike inhibition by dopamine was not precluded by blocking I(h). Consistent with the reduced rate of spike rise, dopamine reduced voltage-gated Na(+) current (I(Na)) amplitude, and tetrodotoxin, at doses that reduced I(Na) as moderately as dopamine, also inhibited spiking. These results provide the first direct evidence that D(1)-type dopamine receptor activation can alter mammalian retinal ganglion cell excitability and demonstrate that dopamine can modulate spikes in these cells by a mechanism different from the presynaptic and postsynaptic means proposed by previous studies. To our knowledge, our results also provide the first evidence that dopamine receptor activation can reduce excitability without altering the temporal precision of spike firing.
Figures



References
-
- Ariano MA, Kang HC, Haugland RP, Sibley DR. Multiple fluorescent ligands for dopamine receptors. II. Visualization in neural tissues. Brain Res. 1991;547:208–222. - PubMed
-
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42:653–663. - PubMed
-
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. - PubMed
-
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. - PubMed
-
- Behrens UD, Wagner HJ. Localization of dopamine D1-receptors in vertebrate retinae. Neurochem Int. 1995;27:497–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
