A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states
- PMID: 19940197
- PMCID: PMC2830115
- DOI: 10.1523/JNEUROSCI.3451-09.2009
A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states
Abstract
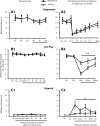
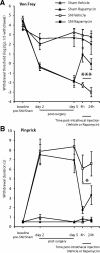
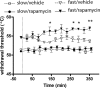
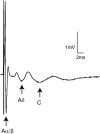
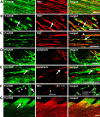
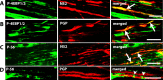
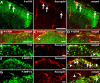
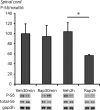
Translational control through the mammalian target of rapamycin (mTOR) is critical for synaptic plasticity, cell growth, and axon guidance. Recently, it was also shown that mTOR signaling was essential for the maintenance of the sensitivity of subsets of adult sensory neurons. Here, we show that persistent pain states, but not acute pain behavior, are substantially alleviated by centrally administered rapamycin, an inhibitor of the mTOR pathway. We demonstrate that rapamycin modulates nociception by acting on subsets of primary afferents and superficial dorsal horn neurons to reduce both primary afferent sensitivity and central plasticity. We found that the active form of mTOR is present in a subpopulation of myelinated dorsal root axons, but rarely in unmyelinated C-fibers, and heavily expressed in the dorsal horn by lamina I/III projection neurons that are known to mediate the induction and maintenance of pain states. Intrathecal injections of rapamycin inhibited the activation of downstream targets of mTOR in dorsal horn and dorsal roots and reduced the thermal sensitivity of A-fibers. Moreover, in vitro studies showed that rapamycin increased the electrical activation threshold of Adelta-fibers in dorsal roots. Together, our results imply that central rapamycin reduces neuropathic pain by acting both on an mTOR-positive subset of A-nociceptors and lamina I projection neurons and suggest a new pharmacological route for therapeutic intervention in persistent pain states.
Figures
Similar articles
-
Tissue plasminogen activator in primary afferents induces dorsal horn excitability and pain response after peripheral nerve injury.Eur J Neurosci. 2004 Jan;19(1):93-102. doi: 10.1046/j.1460-9568.2003.03080.x. Eur J Neurosci. 2004. PMID: 14750967
-
Upregulation and redistribution of ephrinB and EphB receptor in dorsal root ganglion and spinal dorsal horn neurons after peripheral nerve injury and dorsal rhizotomy.Eur J Pain. 2008 Nov;12(8):1031-9. doi: 10.1016/j.ejpain.2008.01.011. Epub 2008 Mar 5. Eur J Pain. 2008. PMID: 18321739
-
Oxytocin actions on afferent evoked spinal cord neuronal activities in neuropathic but not in normal rats.Brain Res. 2005 May 31;1045(1-2):124-33. doi: 10.1016/j.brainres.2005.03.020. Epub 2005 Apr 21. Brain Res. 2005. PMID: 15910770
-
The biological role of galanin in normal and neuropathic states.Prog Brain Res. 2000;129:219-30. doi: 10.1016/S0079-6123(00)29016-X. Prog Brain Res. 2000. PMID: 11098692 Review. No abstract available.
-
Neuropathic pain: a maladaptive response of the nervous system to damage.Annu Rev Neurosci. 2009;32:1-32. doi: 10.1146/annurev.neuro.051508.135531. Annu Rev Neurosci. 2009. PMID: 19400724 Free PMC article. Review.
Cited by
-
Persistent Rheb-induced mTORC1 activation in spinal cord neurons induces hypersensitivity in neuropathic pain.Cell Death Dis. 2020 Sep 12;11(9):747. doi: 10.1038/s41419-020-02966-0. Cell Death Dis. 2020. PMID: 32920594 Free PMC article.
-
Targeting AMPK for the Alleviation of Pathological Pain.Exp Suppl. 2016;107:257-285. doi: 10.1007/978-3-319-43589-3_11. Exp Suppl. 2016. PMID: 27812984 Free PMC article. Review.
-
Multispecies-compatible antitumor effects of a cross-species small-interfering RNA against mammalian target of rapamycin.Cell Mol Life Sci. 2012 Sep;69(18):3147-58. doi: 10.1007/s00018-012-0998-1. Epub 2012 May 5. Cell Mol Life Sci. 2012. PMID: 22562582 Free PMC article.
-
Neuroinflammation and central PI3K/Akt/mTOR signal pathway contribute to bone cancer pain.Mol Pain. 2019 Jan-Dec;15:1744806919830240. doi: 10.1177/1744806919830240. Mol Pain. 2019. PMID: 30717619 Free PMC article.
-
Autophagy impairment in a mouse model of neuropathic pain.Mol Pain. 2011 Oct 24;7:83. doi: 10.1186/1744-8069-7-83. Mol Pain. 2011. PMID: 22023914 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
