A two-subunit bacterial sigma-factor activates transcription in Bacillus subtilis
- PMID: 19940246
- PMCID: PMC2795558
- DOI: 10.1073/pnas.0910006106
A two-subunit bacterial sigma-factor activates transcription in Bacillus subtilis
Abstract
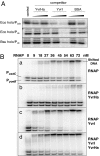
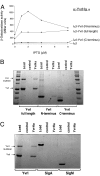
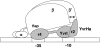
The sigma-like factor YvrI and coregulator YvrHa activate transcription from a small set of conserved promoters in Bacillus subtilis. We report here that these two proteins independently contribute sigma-region 2 and sigma-region 4 functions to a holoenzyme-promoter DNA complex. YvrI binds RNA polymerase (RNAP) through a region 4 interaction with the beta-subunit flap domain and mediates specific promoter recognition but cannot initiate DNA melting at the -10 promoter element. Conversely, YvrHa possesses sequence similarity to a conserved core-binding motif in sigma-region 2 and binds to the N-terminal coiled-coil element in the RNAP beta'-subunit previously implicated in interaction with region 2 of sigma-factors. YvrHa plays an essential role in stabilizing the open complex and interacts specifically with the N-terminus of YvrI. Based on these results, we propose that YvrHa is situated in the transcription complex proximal to the -10 element of the promoter, whereas YvrI is responsible for -35 region recognition. This system presents an unusual example of a two-subunit bacterial sigma-factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Promoter Escape with Bacterial Two-component σ Factor Suggests Retention of σ Region Two in the Elongation Complex.J Biol Chem. 2015 Nov 20;290(47):28575-28583. doi: 10.1074/jbc.M115.666008. Epub 2015 Sep 23. J Biol Chem. 2015. PMID: 26400263 Free PMC article.
-
A previously unidentified sigma factor and two accessory proteins regulate oxalate decarboxylase expression in Bacillus subtilis.Mol Microbiol. 2008 Aug;69(4):954-67. doi: 10.1111/j.1365-2958.2008.06331.x. Epub 2008 Jun 28. Mol Microbiol. 2008. PMID: 18573182 Free PMC article.
-
The YvrI alternative sigma factor is essential for acid stress induction of oxalate decarboxylase in Bacillus subtilis.J Bacteriol. 2009 Feb;191(3):931-9. doi: 10.1128/JB.01435-08. Epub 2008 Dec 1. J Bacteriol. 2009. PMID: 19047353 Free PMC article.
-
Role of the RNA polymerase sigma subunit in transcription initiation.Res Microbiol. 2002 Nov;153(9):557-62. doi: 10.1016/s0923-2508(02)01368-2. Res Microbiol. 2002. PMID: 12455702 Review.
-
How sigma docks to RNA polymerase and what sigma does.Curr Opin Microbiol. 2001 Apr;4(2):126-31. doi: 10.1016/s1369-5274(00)00177-6. Curr Opin Microbiol. 2001. PMID: 11282466 Review.
Cited by
-
Extracytoplasmic Function σ Factors as Tools for Coordinating Stress Responses.Int J Mol Sci. 2021 Apr 9;22(8):3900. doi: 10.3390/ijms22083900. Int J Mol Sci. 2021. PMID: 33918849 Free PMC article. Review.
-
ComWΔ6 Stimulates Transcription of Pneumococcal Competence Genes in vitro.Front Mol Biosci. 2020 May 6;7:61. doi: 10.3389/fmolb.2020.00061. eCollection 2020. Front Mol Biosci. 2020. PMID: 32435654 Free PMC article.
-
Where to begin? Sigma factors and the selectivity of transcription initiation in bacteria.Mol Microbiol. 2019 Aug;112(2):335-347. doi: 10.1111/mmi.14309. Epub 2019 Jun 3. Mol Microbiol. 2019. PMID: 31119812 Free PMC article. Review.
-
Characterization of a protein-protein interaction within the SigO-RsoA two-subunit σ factor: the σ70 region 2.3-like segment of RsoA mediates interaction with SigO.Microbiology (Reading). 2016 Oct;162(10):1857-1869. doi: 10.1099/mic.0.000358. Epub 2016 Aug 23. Microbiology (Reading). 2016. PMID: 27558998 Free PMC article.
-
Promoter Escape with Bacterial Two-component σ Factor Suggests Retention of σ Region Two in the Elongation Complex.J Biol Chem. 2015 Nov 20;290(47):28575-28583. doi: 10.1074/jbc.M115.666008. Epub 2015 Sep 23. J Biol Chem. 2015. PMID: 26400263 Free PMC article.
References
-
- Burgess RR, Anthony L. How sigma docks to RNA polymerase and what sigma does. Curr Opin Microbiol. 2001;4:126–131. - PubMed
-
- Helmann JD, Chamberlin MJ. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. - PubMed
-
- Stragier P, Parsot C, Bouvier J. Two functional domains conserved in major and alternate bacterial sigma factors. FEBS Lett. 1985;187:11–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

