Calreticulin: non-endoplasmic reticulum functions in physiology and disease
- PMID: 19940256
- PMCID: PMC2830142
- DOI: 10.1096/fj.09-145482
Calreticulin: non-endoplasmic reticulum functions in physiology and disease
Abstract
Calreticulin (CRT), when localized to the endoplasmic reticulum (ER), has important functions in directing proper conformation of proteins and glycoproteins, as well as in homeostatic control of cytosolic and ER calcium levels. There is also steadily accumulating evidence for diverse roles for CRT localized outside the ER, including data suggesting important roles for CRT localized to the outer cell surface of a variety of cell types, in the cytosol, and in the extracellular matrix (ECM). Furthermore, the addition of exogenous CRT rescues numerous CRT-driven functions, such as adhesion, migration, phagocytosis, and immunoregulatory functions of CRT-null cells. Recent studies show that topically applied CRT has diverse and profound biological effects that enhance cutaneous wound healing in animal models. This evidence for extracellular bioactivities of CRT has provided new insights into this classically ER-resident protein, despite a lack of knowledge of how CRT exits from the ER to the cell surface or how it is released into the extracellular milieu. Nonetheless, it has become clear that CRT is a multicompartmental protein that regulates a wide array of cellular responses important in physiological and pathological processes, such as wound healing, the immune response, fibrosis, and cancer.-Gold, L. I., Eggleton, P., Sweetwyne, M. T., Van Duyn, L. B., Greives, M. R., Naylor, S.-M., Michalak, M., Murphy-Ullrich, J. E. Calreticulin: non-endoplamic reticulum functions in physiology and disease.
Figures


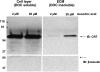




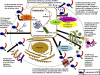
References
-
- Bedard K, Szabo E, Michalak M, Opas M. Cellular functions of endoplasmic reticulum chaperones calreticulin, calnexin, and ERp57. Int Rev Cytol. 2005;245:91–121. - PubMed
-
- Michalak M, Groenendyk J, Szabo E, Gold L I, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417:651–666. - PubMed
-
- Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20:504–511. - PubMed
-
- Obeid M, Tesniere A, Ghiringhelli F, Fimia G M, Apetoh L, Perfettini J L, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

