Designing materials to direct stem-cell fate
- PMID: 19940913
- PMCID: PMC2908011
- DOI: 10.1038/nature08602
Designing materials to direct stem-cell fate
Abstract
Proper tissue function and regeneration rely on robust spatial and temporal control of biophysical and biochemical microenvironmental cues through mechanisms that remain poorly understood. Biomaterials are rapidly being developed to display and deliver stem-cell-regulatory signals in a precise and near-physiological fashion, and serve as powerful artificial microenvironments in which to study and instruct stem-cell fate both in culture and in vivo. Further synergism of cell biological and biomaterials technologies promises to have a profound impact on stem-cell biology and provide insights that will advance stem-cell-based clinical approaches to tissue regeneration.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
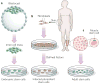

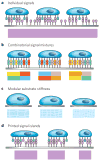
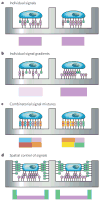
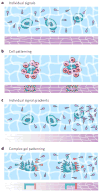

References
-
- Blau HM, Sacco A, Gilbert PM. In: Essentials of Stem Cell Biology. 2. Lanza R, et al., editors. Academic; pp. 249–257. in the press.
-
- Blau HM, Sacco A, Gilbert PM. In: Encyclopedia of Stem Cell Research. Svendsen C, Ebert A, editors. SAGE; in the press.
-
- Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell. 2008;132:544–548. - PubMed
-
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnol. 2005;23:47–55. - PubMed
-
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

