Assignment of 1H, 13C and 15N backbone resonances of Escherichia coli LpxC bound to L-161,240
- PMID: 19941092
- PMCID: PMC3631426
- DOI: 10.1007/s12104-009-9201-5
Assignment of 1H, 13C and 15N backbone resonances of Escherichia coli LpxC bound to L-161,240
Abstract
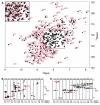
The UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase LpxC catalyzes the committed reaction of lipid A biosynthesis, an essential pathway in Gram-negative bacteria. We report the backbone resonance assignments of the 34 kDa LpxC from Escherichia coli in complex with the antibiotic L-161,240 using multidimensional, multinuclear NMR experiments. The (1)H chemical shifts of complexed L-161,240 are also determined.
Figures

Similar articles
-
Assignment of the 1H, 13C and 15N resonances of the LpxC deacetylase from Aquifex aeolicus in complex with the substrate-analog inhibitor TU-514.J Biomol NMR. 2004 Feb;28(2):201-2. doi: 10.1023/B:JNMR.0000013817.29493.87. J Biomol NMR. 2004. PMID: 14755169 No abstract available.
-
Molecular validation of LpxC as an antibacterial drug target in Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2006 Jun;50(6):2178-84. doi: 10.1128/AAC.00140-06. Antimicrob Agents Chemother. 2006. PMID: 16723580 Free PMC article.
-
Antibacterial agents that target lipid A biosynthesis in gram-negative bacteria. Inhibition of diverse UDP-3-O-(r-3-hydroxymyristoyl)-n-acetylglucosamine deacetylases by substrate analogs containing zinc binding motifs.J Biol Chem. 2000 Apr 14;275(15):11002-9. doi: 10.1074/jbc.275.15.11002. J Biol Chem. 2000. PMID: 10753902
-
UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase (LpxC) inhibitors: a new class of antibacterial agents.Curr Med Chem. 2012;19(13):2038-50. doi: 10.2174/092986712800167374. Curr Med Chem. 2012. PMID: 22414079 Review.
-
Recent Process in the Inhibitors of UDP-3-O-(R-3-hydroxyacyl)-Nacetylglucosamine Deacetylase (LpxC) Against Gram-Negative Bacteria.Mini Rev Med Chem. 2018;18(4):310-323. doi: 10.2174/1389557516666161013120253. Mini Rev Med Chem. 2018. PMID: 27739357 Review.
Cited by
-
Structural basis of the promiscuous inhibitor susceptibility of Escherichia coli LpxC.ACS Chem Biol. 2014 Jan 17;9(1):237-46. doi: 10.1021/cb400067g. Epub 2013 Oct 31. ACS Chem Biol. 2014. PMID: 24117400 Free PMC article.
-
Control of lipopolysaccharide biosynthesis by FtsH-mediated proteolysis of LpxC is conserved in enterobacteria but not in all gram-negative bacteria.J Bacteriol. 2011 Mar;193(5):1090-7. doi: 10.1128/JB.01043-10. Epub 2010 Dec 30. J Bacteriol. 2011. PMID: 21193611 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

