D(2) receptors receive paracrine neurotransmission and are consistently targeted to a subset of synaptic structures in an identified neuron of the crustacean stomatogastric nervous system
- PMID: 19941347
- PMCID: PMC3956453
- DOI: 10.1002/cne.22225
D(2) receptors receive paracrine neurotransmission and are consistently targeted to a subset of synaptic structures in an identified neuron of the crustacean stomatogastric nervous system
Abstract
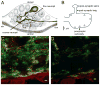
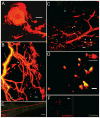
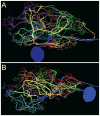
Dopamine (DA) modulates motor systems in phyla as diverse as nematodes and arthropods up through chordates. A comparison of dopaminergic systems across a broad phylogenetic range should reveal shared organizing principles. The pyloric network, located in the stomatogastric ganglion (STG), is an important model for neuromodulation of motor networks. The effects of DA on this network have been well characterized at the circuit and cellular levels in the spiny lobster, Panulirus interruptus. Here we provide the first data about the physical organization of the DA signaling system in the STG and the function of D(2) receptors in pyloric neurons. Previous studies showed that DA altered intrinsic firing properties and synaptic output in the pyloric dilator (PD) neuron, in part by reducing calcium currents and increasing outward potassium currents. We performed single cell reverse transcriptase-polymerase chain reaction (RT-PCR) experiments to show that PD neurons exclusively expressed a type 2 (D(2alphaPan)) DA receptor. This was confirmed by using confocal microscopy in conjunction with immunohistochemistry (IHC) on STG whole-mount preparations containing dye-filled PD neurons. Immunogold electron microscopy showed that surface receptors were concentrated in fine neurites/terminal swellings and vesicle-laden varicosities in the synaptic neuropil. Double-label IHC experiments with tyrosine hydroxylase antiserum suggested that the D(2alphaPan) receptors received volume neurotransmissions. Receptors were further mapped onto three-dimensional models of PD neurons built from Neurolucida tracings of confocal stacks from the IHC experiments. The data showed that D(2alphaPan) receptors were selectively targeted to approximately 40% of synaptic structures in any given PD neuron, and were nonuniformly distributed among neurites.
Figures







Similar articles
-
Dopamine modulates graded and spike-evoked synaptic inhibition independently at single synapses in pyloric network of lobster.J Neurophysiol. 1998 Apr;79(4):2063-9. doi: 10.1152/jn.1998.79.4.2063. J Neurophysiol. 1998. PMID: 9535968
-
Cellular localization of Shab and Shaw potassium channels in the lobster stomatogastric ganglion.Neuroscience. 2004;123(4):919-30. doi: 10.1016/j.neuroscience.2003.08.036. Neuroscience. 2004. PMID: 14751285
-
Crustacean dopamine receptors: localization and G protein coupling in the stomatogastric ganglion.J Neurochem. 2008 Feb;104(4):1006-19. doi: 10.1111/j.1471-4159.2007.05029.x. Epub 2007 Nov 6. J Neurochem. 2008. PMID: 17986222 Free PMC article.
-
Distributed effects of dopamine modulation in the crustacean pyloric network.Ann N Y Acad Sci. 1998 Nov 16;860:155-67. doi: 10.1111/j.1749-6632.1998.tb09046.x. Ann N Y Acad Sci. 1998. PMID: 9928309 Review.
-
Transcriptomic evidence for dense peptidergic networks within forebrains of four widely divergent tetrapods.Curr Opin Neurobiol. 2021 Dec;71:100-109. doi: 10.1016/j.conb.2021.09.011. Epub 2021 Nov 11. Curr Opin Neurobiol. 2021. PMID: 34775262 Review.
Cited by
-
Dopamine-induced oscillations of the pyloric pacemaker neuron rely on release of calcium from intracellular stores.J Neurophysiol. 2011 Sep;106(3):1288-98. doi: 10.1152/jn.00456.2011. Epub 2011 Jun 15. J Neurophysiol. 2011. PMID: 21676929 Free PMC article.
-
Monoaminergic tone supports conductance correlations and stabilizes activity features in pattern generating neurons of the lobster, Panulirus interruptus.Front Neural Circuits. 2015 Oct 20;9:63. doi: 10.3389/fncir.2015.00063. eCollection 2015. Front Neural Circuits. 2015. PMID: 26539083 Free PMC article.
-
Tonic nanomolar dopamine enables an activity-dependent phase recovery mechanism that persistently alters the maximal conductance of the hyperpolarization-activated current in a rhythmically active neuron.J Neurosci. 2011 Nov 9;31(45):16387-97. doi: 10.1523/JNEUROSCI.3770-11.2011. J Neurosci. 2011. PMID: 22072689 Free PMC article.
-
Neuromodulation of neuronal circuits: back to the future.Neuron. 2012 Oct 4;76(1):1-11. doi: 10.1016/j.neuron.2012.09.010. Neuron. 2012. PMID: 23040802 Free PMC article. Review.
-
Cell specific dopamine modulation of the transient potassium current in the pyloric network by the canonical D1 receptor signal transduction cascade.J Neurophysiol. 2010 Aug;104(2):873-84. doi: 10.1152/jn.00195.2010. Epub 2010 Jun 2. J Neurophysiol. 2010. PMID: 20519576 Free PMC article.
References
-
- Agnati LF, Leo G, Zanardi A, Genedani S, Rivera A, Fuxe K, Guidolin D. Volume transmission and wiring transmission from cellular to molecular networks: history and perspectives. Acta Physiol (Oxf) 2006;187:329–344. - PubMed
-
- Barker DL, Kushner PD, Hooper NK. Synthesis of dopamine and octopamine in the crustacean stomatogastric nervous system. Brain Res. 1979;161:99–113. - PubMed
-
- Baro DJ, Cole CL, Harris-Warrick RM. RT-PCR analysis of shaker, shab, shaw, and shal gene expression in single neurons and glial cells. Receptors Channels. 1996;4:149–159. - PubMed
-
- Baro DJ, Levini RM, Kim MT, Willms AR, Lanning CC, Rodriguez HE, Harris-Warrick RM. Quantitative single-cell-reverse transcription-PCR demonstrates that A-current magnitude varies as a linear function of shal gene expression in identified stomatogastric neurons. J Neurosci. 1997;17:6597–6610. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

