Rhodopsin: the functional significance of asn-linked glycosylation and other post-translational modifications
- PMID: 19941415
- PMCID: PMC2881540
- DOI: 10.1080/13816810902962405
Rhodopsin: the functional significance of asn-linked glycosylation and other post-translational modifications
Erratum in
- Ophthalmic Genet. 2010 Mar;31(1):52
Abstract
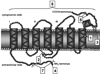
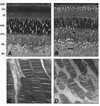
Rhodopsin, the G-protein coupled receptor in retinal rod photoreceptors, is a highly conserved protein that undergoes several types of post-translational modifications. These modifications are essential to maintain the protein's structure as well as its proper function in the visual transduction cycle. Rhodopsin is N-glycosylated at Asn-2 and Asn-15 in its extracellular N-terminal domain. Mutations within the glycosylation consensus sequences of rhodopsin cause autosomal dominant retinitis pigmentosa, a disease that leads to blindness. Several groups have studied the role of rhodopsin's N-linked glycan chains in protein structure and function using a variety of approaches. These include the generation of a transgenic mouse model, study of a naturally occurring mutant animal model, in vivo pharmacological inhibition of glycosylation, and in vitro analyses using transfected COS-1 cells. These studies have provided insights into the possible role of rhodopsin glycosylation, but have yielded conflicting results.
Figures


Similar articles
-
Structure and function in rhodopsin: the role of asparagine-linked glycosylation.Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4024-8. doi: 10.1073/pnas.91.9.4024. Proc Natl Acad Sci U S A. 1994. PMID: 8171029 Free PMC article.
-
Post-translational modifications of the serotonin type 4 receptor heterologously expressed in mouse rod cells.Biochemistry. 2012 Jan 10;51(1):214-24. doi: 10.1021/bi201707v. Epub 2011 Dec 20. Biochemistry. 2012. PMID: 22145929 Free PMC article.
-
Glycosylation of rhodopsin is necessary for its stability and incorporation into photoreceptor outer segment discs.Hum Mol Genet. 2015 May 15;24(10):2709-23. doi: 10.1093/hmg/ddv031. Epub 2015 Jan 30. Hum Mol Genet. 2015. PMID: 25637522 Free PMC article.
-
Rhodopsin and retinitis pigmentosa: shedding light on structure and function.Recept Channels. 2002;8(1):33-50. Recept Channels. 2002. PMID: 12402507 Review.
-
Structure and mechanisms of function of visual system proteins.Membr Cell Biol. 2000;13(2):165-93. Membr Cell Biol. 2000. PMID: 10779170 Review.
Cited by
-
Disease modeling and pharmacological rescue of autosomal dominant Retinitis Pigmentosa associated with RHO copy number variation.medRxiv [Preprint]. 2023 Nov 6:2023.02.27.23286248. doi: 10.1101/2023.02.27.23286248. medRxiv. 2023. Update in: Elife. 2024 Apr 25;12:RP90575. doi: 10.7554/eLife.90575. PMID: 36909455 Free PMC article. Updated. Preprint.
-
The molecular and cellular basis of rhodopsin retinitis pigmentosa reveals potential strategies for therapy.Prog Retin Eye Res. 2018 Jan;62:1-23. doi: 10.1016/j.preteyeres.2017.10.002. Epub 2017 Oct 16. Prog Retin Eye Res. 2018. PMID: 29042326 Free PMC article. Review.
-
Functional compartmentalization of photoreceptor neurons.Pflugers Arch. 2021 Sep;473(9):1493-1516. doi: 10.1007/s00424-021-02558-7. Epub 2021 Apr 20. Pflugers Arch. 2021. PMID: 33880652 Free PMC article. Review.
-
Nonretinoid chaperones improve rhodopsin homeostasis in a mouse model of retinitis pigmentosa.JCI Insight. 2022 May 23;7(10):e153717. doi: 10.1172/jci.insight.153717. JCI Insight. 2022. PMID: 35472194 Free PMC article.
-
The Retinoid and Non-Retinoid Ligands of the Rod Visual G Protein-Coupled Receptor.Int J Mol Sci. 2019 Dec 10;20(24):6218. doi: 10.3390/ijms20246218. Int J Mol Sci. 2019. PMID: 31835521 Free PMC article. Review.
References
-
- Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25:413–422. - PubMed
-
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. - PubMed
-
- Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev. 2000;21:90–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
