Have giant lobelias evolved several times independently? Life form shifts and historical biogeography of the cosmopolitan and highly diverse subfamily Lobelioideae (Campanulaceae)
- PMID: 19941635
- PMCID: PMC2789055
- DOI: 10.1186/1741-7007-7-82
Have giant lobelias evolved several times independently? Life form shifts and historical biogeography of the cosmopolitan and highly diverse subfamily Lobelioideae (Campanulaceae)
Abstract
Background: The tendency of animals and plants to independently develop similar features under similar evolutionary pressures - convergence - is a widespread phenomenon in nature. In plants, convergence has been suggested to explain the striking similarity in life form between the giant lobelioids (Campanulaceae, the bellflower family) of Africa and the Hawaiian Islands. Under this assumption these plants would have developed the giant habit from herbaceous ancestors independently, in much the same way as has been suggested for the giant senecios of Africa and the silversword alliance of Hawaii.
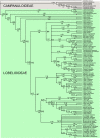
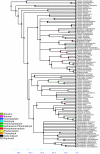
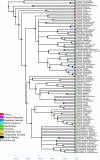
Results: Phylogenetic analyses based on plastid (rbcL, trnL-F) and nuclear (internal transcribed spacer [ITS]) DNA sequences for 101 species in subfamily Lobelioideae demonstrate that the large lobelioids from eastern Africa the Hawaiian Islands, and also South America, French Polynesia and southeast Asia, form a strongly supported monophyletic group. Ancestral state reconstructions of life form and distribution, taking into account phylogenetic uncertainty, indicate their descent from a woody ancestor that was probably confined to Africa. Molecular dating analyses using Penalized Likelihood and Bayesian relaxed clock approaches, and combining multiple calibration points, estimate their first diversification at approximately 25-33 million years ago (Ma), shortly followed by several long-distance dispersal events that resulted in the current pantropical distribution.
Conclusion: These results confidently show that lobelioid species, commonly called 'giant', are very closely related and have not developed their giant form from herbaceous ancestors independently. This study, which includes the hitherto largest taxon sampling for subfamily Lobelioideae, highlights the need for a broad phylogenetic framework for testing assumptions about morphological development in general, and convergent evolution in particular.
Figures







References
-
- Linnaeus C. Systema Naturae. 10. Vol. 1. Laurentii Salvii, Holmiae; 1758. p. 824.
-
- Givnish TJ, Sytsma KJ. Molecular Evolution and Adaptive Radiation. Cambridge: Cambridge University Press; 1997. - PubMed
-
- Futuyma DJ, Edwards SV, John R. Evolution. Massachusetts: Sinauer Associates; 2005.
-
- Hedberg O. Features of afroalpine plant ecology. Stockholm: Almqvist & Wiksell; 1964.
-
- Fries RE, Fries TCE. Die riesen-lobelien Africas. Svensk Botanisk Tidskrift. 1922;16:383–416.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

