Alkyltransferase-mediated toxicity of bis-electrophiles in mammalian cells
- PMID: 19941875
- PMCID: PMC2813384
- DOI: 10.1016/j.mrfmmm.2009.11.006
Alkyltransferase-mediated toxicity of bis-electrophiles in mammalian cells
Abstract
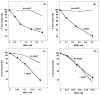
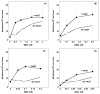
The primary function of O(6)-alkylguanine-DNA alkyltransferase (AGT) is to maintain genomic integrity in the face of damage by both endogenous and exogenous alkylating agents. However, paradoxically, bacterial and mammalian AGTs have been shown to increase cytotoxicity and mutagenicity of dihaloalkanes and other bis-electrophiles when expressed in bacterial cells. We have extended these studies to mammalian cells using CHO cells that lack AGT expression and CHO cells stably transfected with a plasmid that expresses human AGT. The cytotoxicity of 1,2-dibromoethane, dibromomethane and epibromohydrin was significantly increased by the presence of AGT but cytotoxicity of butadiene diepoxide was not affected. Mutations caused by these agents were assessed using hypoxanthine-guanine phosphoribosyltransferase (HPRT) as a reporter gene. There was a small (c. 2-3-fold) but statistically significant AGT-mediated increase in mutations caused by 1,2-dibromoethane, dibromomethane and epibromohydrin. Analysis of the mutation spectrum induced by 1,2-dibromoethane showed that the presence of AGT also altered the types of mutations with an increase in total base substitution mutants due to a rise in transversions at both G:C and A:T sites. AGT expression also led to mutations arising from the transcribed strand, which were not seen in cells lacking AGT. Although the frequency of deletion mutations was decreased by AGT expression, the formation of large deletions (> or = 3 exons) was increased. This work demonstrates that interaction of AGT with some bis-electrophiles can cause mutagenicity and diminished cell survival in mammalian cells. It is consistent with the hypothesis that DNA-AGT cross-links, which have been characterized in experiments with purified AGT protein and such bis-electrophiles, can be formed in mammalian cells.
Copyright 2009 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest
Figures
Similar articles
-
Multifaceted roles of alkyltransferase and related proteins in DNA repair, DNA damage, resistance to chemotherapy, and research tools.Chem Res Toxicol. 2011 May 16;24(5):618-39. doi: 10.1021/tx200031q. Epub 2011 Apr 28. Chem Res Toxicol. 2011. PMID: 21466232 Free PMC article. Review.
-
O6-alkylguanine-DNA alkyltransferase has opposing effects in modulating the genotoxicity of dibromomethane and bromomethyl acetate.Chem Res Toxicol. 2004 Jun;17(6):742-52. doi: 10.1021/tx049958o. Chem Res Toxicol. 2004. PMID: 15206895
-
Activation of bis-electrophiles to mutagenic conjugates by human O6-alkylguanine-DNA alkyltransferase.Chem Res Toxicol. 2004 Jul;17(7):972-82. doi: 10.1021/tx049897u. Chem Res Toxicol. 2004. PMID: 15257623
-
Effect of O6-alkylguanine-DNA alkyltransferase on genotoxicity of epihalohydrins.Environ Mol Mutagen. 2009 Jul;50(6):502-14. doi: 10.1002/em.20491. Environ Mol Mutagen. 2009. PMID: 19472322 Free PMC article.
-
Principles of covalent binding of reactive metabolites and examples of activation of bis-electrophiles by conjugation.Arch Biochem Biophys. 2005 Jan 15;433(2):369-78. doi: 10.1016/j.abb.2004.07.035. Arch Biochem Biophys. 2005. PMID: 15581593 Review.
Cited by
-
Multifaceted roles of alkyltransferase and related proteins in DNA repair, DNA damage, resistance to chemotherapy, and research tools.Chem Res Toxicol. 2011 May 16;24(5):618-39. doi: 10.1021/tx200031q. Epub 2011 Apr 28. Chem Res Toxicol. 2011. PMID: 21466232 Free PMC article. Review.
-
Synthesis and Characterization of Site-Specific O6 -Alkylguanine DNA-Alkyl Transferase-Oligonucleotide Crosslinks.Curr Protoc Nucleic Acid Chem. 2019 Mar;76(1):e74. doi: 10.1002/cpnc.74. Epub 2019 Jan 18. Curr Protoc Nucleic Acid Chem. 2019. PMID: 30657645 Free PMC article.
-
In vivo roles of conjugation with glutathione and O6-alkylguanine DNA-alkyltransferase in the mutagenicity of the bis-electrophiles 1,2-dibromoethane and 1,2,3,4-diepoxybutane in mice.Chem Res Toxicol. 2013 Nov 18;26(11):1765-74. doi: 10.1021/tx4003534. Epub 2013 Nov 6. Chem Res Toxicol. 2013. PMID: 24191644 Free PMC article.
-
DNA-protein crosslinks processed by nucleotide excision repair and homologous recombination with base and strand preference in E. coli model system.Mutat Res. 2013 Jan-Feb;741-742:1-10. doi: 10.1016/j.mrfmmm.2013.02.005. Epub 2013 Mar 15. Mutat Res. 2013. PMID: 23500083 Free PMC article.
-
Detection and characterization of 1,2-dibromoethane-derived DNA crosslinks formed with O(6) -alkylguanine-DNA alkyltransferase.Angew Chem Int Ed Engl. 2013 Dec 2;52(49):12879-82. doi: 10.1002/anie.201307580. Epub 2013 Oct 15. Angew Chem Int Ed Engl. 2013. PMID: 24130045 Free PMC article.
References
-
- Komsta E, Chu I, Secours VE, Valli VE, Villeneuve DC. Results of a short-term toxicity study for three organic chemicals found in Niagara River drinking water. Bull Environ Contam Toxicol. 1988;41:515–522. - PubMed
-
- Rannug U. Genotoxic effects of 1,2-dibromoethane and 1,2-dichloroethane. Mutat Res. 1980;76:269–295. - PubMed
-
- Liu L, Pegg AE, Williams KM, Guengerich FP. Paradoxical enhancement of the toxicity of 1,2-dibromoethane by O6-alkylguanine-DNA alkyltransferase. J Biol Chem. 2002;277:37920–37928. - PubMed
-
- Osterman-Golkar S, Hussain S, Walles S, Anderstam B, Sigvardsson K. Chemical reactivity and mutagenicity of some dihalomethanes. Chem Biol Interact. 1983;46:121–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

