Injectable in situ cross-linking hydrogels for local antifungal therapy
- PMID: 19942285
- PMCID: PMC2813953
- DOI: 10.1016/j.biomaterials.2009.11.016
Injectable in situ cross-linking hydrogels for local antifungal therapy
Abstract
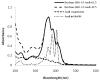
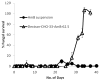
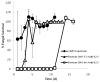
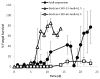
Invasive fungal infections can be devastating, particularly in immunocompromised patients, and difficult to treat with systemic drugs. Furthermore, systemic administration of those medications can have severe side effects. We have developed an injectable local antifungal treatment for direct administration into existing or potential sites of fungal infection. Amphotericin B (AmB), a hydrophobic, potent, and broad-spectrum antifungal agent, was rendered water-soluble by conjugation to a dextran-aldehyde polymer. The dextran-aldehyde-AmB conjugate retained antifungal efficacy against Candida albicans. Mixing carboxymethylcellulose-hydrazide with dextran-aldehyde formed a gel that cross-linked in situ by formation of hydrazone bonds. The gel provided in vitro release of antifungal activity for 11 days, and contact with the gel killed Candida for three weeks. There was no apparent tissue toxicity in the murine peritoneum and the gel caused no adhesions. Gels produced by entrapment of a suspension of AmB in CMC-dextran without conjugation of drug to polymers did not release fungicidal activity, but did kill on contact. Injectable systems of these types, containing soluble or insoluble drug formulations, could be useful for treatment of local antifungal infections, with or without concurrent systemic therapy.
(c) 2009 Elsevier Ltd. All rights reserved.
Figures







References
-
- Naeger-Murphy N, Pile JC. Clinical Indications for Newer Antifungal Agents. J Hosp Med. 2009;4(2):102–111. - PubMed
-
- Ruszczak Z, Friess W. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv Drug Deliver Rev. 2003;55(12):1679–1698. - PubMed
-
- Zilberman M, Elsner JJ. Antibiotic-eluting medical devices for various applications. J Control Release. 2008;130(3):202–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

